
Project Gutenberg's The Preservation of Antiquities, by Friedrich Rathgen
This eBook is for the use of anyone anywhere in the United States and most
other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms of
the Project Gutenberg License included with this eBook or online at
www.gutenberg.org. If you are not located in the United States, you'll have
to check the laws of the country where you are located before using this ebook.
Title: The Preservation of Antiquities
A Handbook for Curators
Author: Friedrich Rathgen
Translator: George A. Auden
Harold A. Auden
Release Date: September 14, 2014 [EBook #46851]
Language: English
Character set encoding: ISO-8859-1
*** START OF THIS PROJECT GUTENBERG EBOOK THE PRESERVATION OF ANTIQUITIES ***
Produced by Chris Curnow, Sonya Schermann and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)
The cover image was created by the transcriber and is placed in the public domain.
Time, which antiquates antiquities, and hath an art to make dust of all things, hath yet spared these minor monuments.
(Sir Thomas Browne, Hydriotaphia, cap. v.)
THE PRESERVATION
OF ANTIQUITIES
A HANDBOOK FOR CURATORS
TRANSLATED, BY PERMISSION OF THE AUTHORITIES OF THE
ROYAL MUSEUMS, FROM THE GERMAN OF
Dr FRIEDRICH RATHGEN
Director of the Laboratory of the Royal Museums,
Berlin
BY
GEORGE A. AUDEN, M.A., M.D. (Cantab.)
AND
HAROLD A. AUDEN, M.Sc. (Vict.), D.Sc. (Tübingen)
CAMBRIDGE:
at the University Press
1905
CAMBRIDGE UNIVERSITY PRESS WAREHOUSE,
C. F. CLAY, Manager.
London: AVE MARIA LANE, E.C.
Glasgow: 50, WELLINGTON STREET.

Leipzig: F. A. BROCKHAUS.
New York: THE MACMILLAN COMPANY.
Bombay and Calcutta: MACMILLAN AND CO., Ltd.
[All Rights reserved.]
The increasing recognition of the importance of the preservation of antiquities justifies the publication of a handbook dealing with this subject. As far as I can ascertain, with the exception of a short article[1] for which I am myself responsible, only one work has appeared which covers the whole field—the “Merkbuch[2]” prepared by Dr Voss at the request of the Government. But as this book only gives a selection of the known methods of preservation, the need of a more comprehensive publication will scarcely be denied.
In spite of my ten years’ experience in the special Laboratory of the Royal Museums and the frequent opportunities of learning the methods in use elsewhere, which the journeys and correspondence arising out of my duties have given me during this period, I do not feel competent to produce a review of these various methods [vi] which will be at once exhaustive and sufficiently critical. There are several reasons for this. In the first place the individual methods have been but rarely published, and even then through the most varied literary media; often they are only casually mentioned in articles dealing with anthropological or historical subjects. On the other hand, the value of an object to be dealt with may prohibit an attempt at treatment, the success of which is not assured. My own experience has been gained by trials with objects chiefly from the Egyptian section, but also to some extent from the Antiquarian and Numismatic departments of the Royal Museums.
This deficiency can only be remedied by a work such as that now offered to the public, and it is to be hoped that this handbook will stimulate the Curators of State, Municipal and Societies’ Collections, as well as private collectors and others interested in the subject, to make public their further experiences in this field of archaeology. I take this opportunity, therefore, of expressing the hope that I may receive other communications bearing upon the subject and may thus perhaps at some future date be able to produce a more complete work.
In using the book it will be noticed that for the proper understanding of the first portion, which deals with the causes of destruction, a certain amount of chemical knowledge is assumed. In the second portion, [vii] however, the methods of preservation are treated from a more elementary standpoint, and the simple apparatus and manipulations required are so described that the treatment may be readily carried out by those who are unfamiliar with chemical methods.
In conclusion, I take this opportunity of expressing my thanks to all those who have given their help, and especially to Dr Otto Olshausen for his continued interest in the work of the Museum Laboratory and in the production of this handbook. Especially am I indebted to his extensive knowledge of anthropological literature for many references which would otherwise have escaped my notice. [viii]
Dr Rathgen has, in his preface, stated the aim of this handbook, and it is with a desire to further this aim that we have prepared an English translation.
Claiming but limited experience in this field of research we have only added such explanatory notes as seem in some way to bear upon the subject or likely to be useful in a handbook of this kind (viz. the method of taking squeezes, Appendix A, and a few footnotes which are signed and enclosed in square brackets). We take this opportunity of thanking Dr Rathgen for his interest in our undertaking, for his kindness in supplying much additional matter which did not appear in the German edition, and also for the loan of the blocks for Figs. 22 and 23. Figs. 7, 9-12, 30-33, and 37, are from photographs of objects treated by ourselves.
Our thanks are especially due to Dr W. A. Caspari, of the National Physical Laboratory, for his invaluable help in the revision of the translation, and for his advice and suggestions in reference to the more technical aspect of the work.
York,
December 1904.
| PAGE | |
| Literature | xiii |
| Part I. | |
| The changes undergone by antiquities in earth and in air | 1 |
| Limestone and clay | 2 |
| Iron | 7 |
| Bronze and copper | 15 |
| Silver | 49 |
| Lead | 53 |
| Tin | 53 |
| Gold | 53 |
| Glass | 54 |
| Organic substances | 54 |
| Part II. | |
| The preservation of antiquities | 56 |
| i. Preservation of objects composed of inorganic substances | |
| a. Limestone | 56 |
| b. Marble and alabaster | 74 |
| c. Earthenware | 74 |
| d. Slightly baked or unbaked clay | 81 |
| e. Fayence | 86 |
| f. Stucco and Nile-mud | 87 |
| g. Sandstone and granite | 87 |
| Appendix: Cement for earthenware. Restorations | 87 |
| h. Iron | 89 |
| 1. Methods of preserving objects of iron without removal of the rust | 89 |
| [x] 2. Preservation by steeping and subsequent impregnation | 92 |
| 3. Preservation by removal of the rust | 102 |
| 4. Preservation of medieval iron objects | 119 |
| i. Bronze and copper | 120 |
| A. Methods of impregnation | 122 |
| B. Preservation by reduction | 125 |
| Reduction of oxidized copper coins | 140 |
| Cleaning copper coins with melted lead | 143 |
| C. Preservation by exclusion of air | 144 |
| Appendix: Method of bringing out worn lettering upon coins | 146 |
| j. Silver | 148 |
| k. Lead and tin | 149 |
| l. Gold | 150 |
| m. Glass and enamel | 151 |
| ii. Preservation of organic substances. | |
| n. Bones, horns, ivory | 151 |
| o. Leather | 152 |
| p. Textile fabrics, hair | 153 |
| q. Feathers | 154 |
| r. Papyrus | 154 |
| s. Wood | 156 |
| 1. Dry preservation | 156 |
| 2. Preservation in liquids | 159 |
| Protection against wood-worms, etc. | 160 |
| Preservation and cleaning of coloured objects of wood | 161 |
| t. Amber | 162 |
| Care of antiquities after preservative treatment | 162 |
| Concluding remarks | 164 |
| Appendix A. Method of taking squeezes of inscriptions | 166 |
| Appendix B. Zapon | 168 |
| Index | 171 |
| FIG. | PAGE | ||||
| 1. | Limestone block with well-preserved surface | 3 | |||
| 2. | Limestone block with pitted surface | 3 | |||
| 3. | Limestone block showing destruction of surface | 4 | |||
| 4. | Potsherd showing saline efflorescence | 5 | |||
| 5. | Pottery showing sodium nitrate efflorescence | 6 | |||
| 6. | Portion of horse-trappings showing blue and green patina | 35 | |||
| 7. | Head of Osiris showing advanced condition of warty patina | 38 | |||
| 8. | Etruscan mirror showing warty patina | 40 | |||
| 9. | Bronze figure showing destructive patina | 42 | |||
| 10. | 43 | ||||
| 11. | The same after treatment (Finkener’s method) | 44 | |||
| 12. | 45 | ||||
| 13. | Gay-Lussac’s burette | 62 | |||
| 14. | Air-pump fixed to water-tap | 68 | |||
| 15. | Apparatus for impregnation by extraction of air | 69 | |||
| 16. | Assyrian clay tablet showing incrustation | 79 | |||
| 17. | The same after treatment | 79 | |||
| 18. | |||||
| 19. | Assyrian clay tablet before and after treatment | 80 | |||
| 20. | |||||
| 21. | |||||
| 22. | Babylonian clay cone before and after treatment | 82 | |||
| 23. | 83 | ||||
| 24. | Water-bath for iron objects | 94 | |||
| 25. | Iron sword treated by Blell’s method | 108 | |||
| 26. | Iron spear-head treated by Blell’s method | 109 | [xii] | ||
| 27. | Iron fibula treated by Blell’s method | 109 | |||
| 28. | Application of Krefting’s method | 111 | |||
| 29. | Iron spear-head treated by Krefting’s method | 112 | |||
| 30. | Iron pin before and after treatment by Krefting’s method | 113 | |||
| 31. | |||||
| 32. | Iron object before and after treatment by Krefting’s method | 114 | |||
| 33. | |||||
| 34. | Piece of iron sword-blade with inscription revealed by Krefting’s method | 116 | |||
| 35. | Iron sheath after treatment by combination of Blell’s and Krefting’s method | 117 | |||
| 36. | Hammer-heads for removal of bronze incrustations | 120 | |||
| 37. | Osiris showing cracking and destructive patina | 123 | |||
| 38. | Boeotian bridle showing cracked patina | 124 | |||
| 39. | Bronze bull showing warty patina | 132 | |||
| 40. | The same after reduction by Finkener’s method | 133 | |||
| 41. | Bronze axe-blade before treatment by Finkener’s method | 134 | |||
| 42. | The same after treatment by Finkener’s method | 135 | |||
| 43. | Reverse side of same after treatment | 136 | |||
| 44. | Dagger-sheath before treatment by Finkener’s method | 137 | |||
| 45. | Dagger-sheath after treatment, showing design | 137 | |||
| 46. | Roman coins before treatment by Krefting’s method | 142 | |||
| 47. | Roman coins after treatment by Krefting’s method | 143 | |||
| 48. | Method of mounting objects in air-tight damp-proof cases | 145 |
Aarböger for nordisk Oldkyndighed og Historie, udgivne af det kongelige nordiske Oldskrift-Selskab. Copenhagen.
Aarsberetning fra Foreningen till norske Fortidsmindesmaerkers Bevaring. Christiania.
Annalen der Chemie und Pharmacie. Edited by Wöhler, Liebig and Kopp. Since 1873: Liebig’s Annalen der Chemie.
Antiquarisk Tidsskrift, udgivet af det kongelige nordiske Oldskrift-Selskab. Copenhagen 1843-63.
Archaeological Journal. London.
Atti della Reale Accademia dei Lincei. Rome.
Berg- und hüttenmännische Zeitung. Leipzig.
Bibra, E. v. Die Bronzen und Kupferlegirungen der alten und ältesten Völker. Erlangen 1869.
Bibra, E. v. Ueber alte Eisen- und Silberfunde. Nürnberg and Leipzig 1873.
Bischoff, C. Das Kupfer und seine Legirungen. Berlin 1865.
Blätter für Münzkunde. Hannoversche numismatische Zeitschrift. Edited by H. Grote. Leipzig.
Chemiker-Zeitung (Dr G. Krause). Cöthen.
Chemisches Centralblatt (Arendt) Hamburg and Leipzig.
Christiania Videnskabs-Selskabs Forhandlinger. Christiania.
Comptes rendus hebdomadaires des séances de l’Académie des sciences, publ. p. les secrétaires perpétuels. Paris.
Dingler’s Polytechnisches Journal. Stuttgart.
Finska Fornminnesföreningens Tidskrift. Helsingfors.
Finskt Museum. Finska Fornminnesföreningens Månadsblad. Helsingfors.
Friedel, E. Eintheilungsplan des Märkischen Provinzialmuseums der Stadt Berlin. 6th issue. Berlin 1882.
Graham-Otto’s Ausführliches Lehrbuch der Chemie. 5th Edition. Anorgan. Chemie von H. Michaelis. Brunswick 1878-89.
Hauenstein, H. Die Kessler’schen Fluate. 2nd Edition. Berlin 1895.
Journal für praktische Chemie. Edited by Erdmann. Leipzig,
[xiv] Journal of the Chemical Society. London.
Keim, A. Technische Mittheilungen für Malerei. Munich.
Kongl. Vitterhets Historie och Antiqvitets Akademiens Månadsblad. Stockholm.
Kröhnke, Chemische Untersuchungen an vorgeschichtlichen Bronzen Schleswig-Holsteins. Dissertation. Kiel 1897.
Layard. Discoveries in the ruins of Nineveh and Babylon. London 1853.
Lepsius, C. R. Denkmäler aus Aegypten und Aethiopien. Berlin 1849-59.
Lueger, O. Lexikon der gesamten Technik. Stuttgart 1894.
Merkbuch, Alterthümer aufzugraben und aufzubewahren. Herausgeg. auf Veranlassung des Herrn Ministers der geistlichen, Unterrichts- u. Medizinal-Angelegenheiten. 2nd Edition. Berlin 1894.
Mittheilungen der naturforschenden Gesellschaft in Bern. Bern.
Mittheilungen aus der Sammlung der Papyrus Erzherzog Bainer. Vienna 1887-1889.
Morgan, J. de, Fouilles à Dahchour Mars-Juin 1894. Vienna 1895.
Muspratt’s theoretische, praktische u. analytische Chemie. 4th Edition. Brunswick 1883.
Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefakten-Kunde, edited by K. C. von Leonhard and H. G. Bronn. Stuttgart.
Polytechnisches Centralblatt. Leipzig 1835-75.
Polytechnisches Centralblatt. (Geitel.) Organ der polytechn. Gesellschaft zu Berlin. Berlin 1888.
Prometheus, edited by Dr O. N. Witt. Berlin.
Publications de la société pour la recherche et la conservation des monuments historiques dans le grandduché de Luxembourg. Luxemburg.
J. J. Rein, Japan. Nach Reisen und Studien im Auftrage der Königl. Preuss. Regierung. 2 Vols. Leipzig 1881-1886.
Revue archéologique, publiée sous la direction de MM. A. Bertrand et G. Perrot. Paris.
Schliemann, H., Ilios. Leipzig 1881.
Simon, E., Ueber Rostbildung und Eisenanstriche. Berlin 1896.
Sitzungsberichte der Alterthumsgesellschaft Prussia in Königsberg.
Verhandlungen der Berliner Anthropologischen Gesellschaft. Berlin.
Verhandlungen des Vereins zur Beförderung des Gewerbefleisses in Preussen. Berlin.
Zeitschrift für Numismatik. Edited by A. v. Sallet. Berlin.
Zeitschrift für anorganische Chemie.
Zeitschrift für Ethnologie. Berlin.
The greater number of those objects of antiquity which are composed of inorganic materials, such as limestone, earthenware, and metals, owe the commencement of any alteration in their character to the situation in which they are discovered, since they are buried in ground which has been at some period damp or wet, and has contained, moreover, salts soluble in water. Amongst these salts the most usual is sodium chloride (common salt), but this is mostly accompanied by potassium chloride, potassium sulphate, magnesium chloride, and calcium sulphate; in short, by those soluble salts which are found in sea-water. In the fine pores of Egyptian antiquities, especially, such salts occur, and their presence is readily explained by the fact that the land of Egypt was originally a sea-bottom.
The presence of salt in the soil of Egypt has been known for a considerable period. Thus Karabacek[3], quoting from Volney’s “Travels in Syria and Egypt” (Jena, 1788, I. p. 13):
“Wherever one digs one finds brackish water containing soda, sea-salt, and a small quantity of saltpetre. Indeed, when a garden has been flooded for irrigation [2] purposes, crystals of salt make their appearance on the surface after the water has evaporated or has been soaked up by the soil.”
In the dry climate of Egypt, objects saturated with salt keep better after their removal from the ground than in our climate, where the variations in the temperature and in the hygroscopic condition of the air produce a partial deliquescence in wet weather, and in dry weather a re-formation of crystals. The continued alternation of these processes gradually loosens the surface of limestone or earthenware, or induces certain chemical changes in objects of metal and in both cases leads to their destruction.
The series of changes are particularly well illustrated by the Egyptian grave of Meten[4], the stones from which are now in the Royal Museum in Berlin. The three illustrations here given show: (1) an undecayed block of limestone, (2) a block with pitted surface, and (3) a block the surface of which was formerly covered with hieroglyphics, but which is now totally destroyed by flaking. The blocks of the latter kind were found in the lowest layer, or lowest but one, while those blocks which were above were the best preserved. As the amount of salt present scarcely varied, these specimens [4] offer a striking illustration of the greater influence of moisture in the deeper soil than at the higher levels.

Fig. 2.
Limestone block with pitted surface.

Fig. 3.
Limestone block showing destruction of surface.
Baked clay, particularly that of Egyptian ostraca (i.e. fragments of pottery showing inscriptions), exhibits similar changes, as is shown in the accompanying illustrations. The surface of some fragments is found to be almost completely covered with a layer of salt, which, apart from impurities of clay and dust and remains of the black lettering, consists of almost pure sodium chloride; only a trace of magnesium sulphate being found on analysis.
In contrast with this very loose superficial incrustation, the inner portions of the ostracon contained considerable quantities of sulphates. Figure 4 represents a fragment [5] with granular efflorescences of sodium chloride, and also fine needles of magnesium sulphate[5]. As a general rule the amount of salt is small compared with the bulk of clay or limestone: thus it was found by titration that three separate fragments contained 0·13, 0·20, and 0·48% calculated as sodium chloride, and in one series the average of 16 fragments was 0·13%. But the percentage of sodium chloride has often been found higher, more especially in larger objects of baked clay, being in one instance as high as 2·3%. The disintegration of the surface is due to the mechanical action of moisture which results in the scaling off of portions of the [6] surface. This does not however exclude a chemical action of the salts upon the clay, especially when this has been only slightly baked. Thus by merely washing such fragments in cold distilled water, not only sodium and magnesium compounds but also those of aluminium and calcium may be removed. The soft powdery patches, which some limestones show instead of scales, are also evidences of chemical action; thus in one case a cuneiform tablet[6] of dolomitic stone showed decomposition at those spots where the salt was firmly deposited as an incrustation, and here the stone, elsewhere smooth and hard, was found, on washing away the salt, to be soft and porous.

Fig. 4.
Potsherd showing saline efflorescence of sodium chloride and
magnesium sulphate.
Although, as has been already remarked, sodium chloride generally constitutes the bulk of the salts present, and only in rare cases, as I have for instance shown in an Egyptian Fayence and in several Greek clay vases, is the amount of sulphates greater, yet there are in collections clay objects (Fig. 5) covered with needles of sodium nitrate[7] (Chili saltpetre) where [7] the nitric acid has been contributed by the decomposition of organic substances; and here the presence of nitrates proves inimical to antiquities just in the same way as a coating of limewash may be seen to be destroyed by the so-called wall-saltpetre [8].

Fig. 5.
Pottery showing efflorescence of sodium nitrate.
If in some cases it may be uncertain whether the destruction of antiquities of limestone or earthenware has been due to mechanical or to chemical influences, this uncertainty is excluded in the case of metallic objects, of which those of bronze and iron chiefly come under the notice of the antiquary.
From the first piece of metallic iron which he possessed man must have soon become acquainted with its untoward property of rusting, but even at the present day opinions differ as to the origin of rust, and the cause of its rapid spreading. It has long been known with certainty that iron containing but little carbon (wrought iron) rusts with greater ease than iron which is rich in carbon (cast iron or steel), and that the rust is a compound of iron with hydrogen and oxygen (hydroxide). That rust is of variable composition may be inferred from the variations of shade from yellow to dark brown which are met with.
Widely different views are held on the question of the production of rust. Some[9] maintain that iron rusts only in the presence of water containing free oxygen and carbonic acid (CO2) in solution, a ferrous bicarbonate being first formed; the bicarbonate is then converted into ferrous carbonate, which finally yields the hydrate with evolution of [8] carbonic acid. This carbonic acid continues to attack further areas of metallic iron. Others[10] maintain that, while the formation of rust may proceed as described, carbonic acid is not necessary, and that free oxygen alone causes rusting when atmospheric moisture is condensed upon the surface of iron. That iron remains free from rust when in a solution of caustic potash or soda is said to be due to the absence of free oxygen and not to the removal of carbonic acid. Spennrath holds, in opposition to the opinion of Axel Krefting[11], that rust once formed cannot act as an oxidising agent, except by virtue of its power of condensing water and retaining it in its pores. Similarly E. Simon finds the chief cause of the corroding action of rust in the property of absorption, that is surface-condensation of gases. This condition is comparable to that of liquefaction, and produces rapid chemical action. Under certain circumstances ferrous hydrate is formed instead of ferric hydrate, particularly when iron is subjected to vibrations, as Tolomei [12] has observed in iron rails etc. Stapff[13] believes that mixtures of ferric hydrate with ferroso-ferric oxide, which possess a similar composition to forge scale, are formed under the influence of thermal waters. According to Irvine[14] rusting proceeds rapidly when two kinds of iron, such as cast and wrought, are in contact, since their electro-chemical relations may result in a voltaic couple. The electric current brings about the decomposition of the water, and the evolved hydrogen, being in the nascent state, combines with the nitrogen dissolved in the water to form ammonia, as had [9] been previously observed by Akermann[15]. Similarly, electric currents are said to be caused by the contact of ferroso-ferric oxide with metallic iron, thus causing a further oxidation of the iron[16].
[10] The presence of certain neutral salts, especially sodium chloride (common salt), has a very marked influence on the destruction of iron[17].
When iron filings are exposed to air and moisture, oxidation takes place; the action is, however, according to Krefting, far more intense in the presence of an alkaline chloride. A mixture of iron filings and sodium chloride exposed to moisture is converted in a few days into a black powder which has the following composition:—11·4% FeO, 80·0% Fe2O3, 8·6% H2O, thus resembling the “iron-black” of Lemery; on extraction with water the filtrate is found to be alkaline [11] and to possess a tallow-like smell[18]. Without entering further into Krefting’s researches, we will quote the hypothesis with which he concludes:
“The iron probably combines with small quantities of chlorine from the sodium chloride, causing alternate reduction and oxidation, and this, owing to the ease with which iron salts pass from one stage of oxidation to another, very soon gives a visible result in the formation of rust:
Fe + 2NaCl = FeCl2 + 2Na
2Na + 2H2O = 2NaOH + H2.”
If these results be compared with observations made upon the condition of iron objects which have been excavated, it is evident that these are in many cases exposed to the action of the air to a lesser extent while buried, and that their decomposition will advance more rapidly when they have been withdrawn from their protective covering of earth. The condition of the objects differs according to the kind of iron, the length of time during which they have been buried, and the character of the soil in which they are found. In one place objects are found covered with a slight layer of rust only, in another with a thicker layer, in another there remains but a small core of metal, or even none at all, or the layer of rust may be intermingled with particles of earth or clay. The rust may be uniform in colour and hardness in one case, and in another soft areas, generally light in colour, may alternate with darker, harder patches, while frequently the harder layer is found below the lighter and softer, etc.—conditions which depend on the occurrence of the various iron compounds. The [12] behaviour of all, however, when placed in collections, even in the driest of rooms, is the same; all sooner or later undergo change, and portions of rust become detached, until in the course of time every trace of the original metallic core is oxidised. A closer inspection generally shows in these cases small brownish, glistening bubbles[19] which prove, when touched, to be drops consisting of chlorine compounds of iron surrounded and permeated with oxides. Krefting[20] gives as the average of a series of analyses of the rust on northern antiquities the following composition:
| Ferric oxide | 7·05 | ||
| Ferrous oxide | 12·7 | ||
| Carbonic acid | 3·9 | ||
| Calcium oxide | 0·58 | ||
| Magnesium oxide | 0·09 | ||
| Ferrous chloride | 0·260 | ||
| Calcium chloride | 0·280 | ||
| Magnesium chloride | 0·023 | 0·61% Soluble salts. | |
| Potassium chloride | 0·018 | ||
| Sodium chloride | 0·027 | ||
| Water chemically combined | 8·0 | ||
| Moisture | 1·50 | ||
| Organic matter | 0·97 |
Thus the chief part in this rapid decomposition is played by the chlorine compounds, as indeed was previously determined[21] by the experimental proofs already given. If ferrous chloride is present the further decompositions can be explained by such equations as those given by Olshausen[22].
[13]
6FeCl2 + 3O = Fe2O3 +
2Fe2Cl6;
2Fe2Cl6 + 2Fe = 6FeCl2.
The equations do not claim to give a complete statement of the reactions, for other reactions take place at the same time; thus ferric hydrates and carbonates and perhaps also intermediate compounds of oxygen and chlorine occur; they show however that in the oxidation of ferrous chloride, oxides and ferric chloride are produced, so that new and hitherto intact particles of the metal continually react with the ferric chloride.
In many cases the action of the chlorine is not only seen in objects placed in a collection, but also in freshly excavated objects. Not infrequently iron objects are found which are covered with large hard blisters, and are thus more or less deformed. The interior of these blisters consists of a mixture of ferrous chloride with oxides, but the shell has become so hard by complete oxidation that it can only be removed with hammer and chisel.
Iron objects found in peat differ from these chlorine-containing specimens which are found in soil, and although sometimes much corroded, many are well preserved. Blell[23] is of the opinion that if peat is free from tannic acid, the finds will be well preserved, while the theory advanced in the Merkbuch[24] is that tannic acid acts as a preservative. The latter view is probably the more correct, for although ordinary tannic acid seldom occurs in peat, yet peat contains a series of compounds which are tanning agents, such as ulmic, humic, and crenic acids. These form iron compounds which, being insoluble in water, protect the metallic iron beneath from further action. If, however, the peat contains sulphates, and especially if it contains free sulphuric acid, only much corroded iron is likely to be found. Moreover the physical condition [14] of the peat may vary; thus it may be dry or damp or even submerged under water, and this variation will exercise some influence upon the condition of the iron.
Iron objects which are covered with the black, so-called “noble” rust (Edel-rost) usually prove very stable. This, like forge-scale, is a ferroso-ferric compound in which there is a preponderance of ferrous oxide where it is in contact with the metallic iron, and of ferric oxide in the outer layer. “Noble” rust is probably in nearly all instances the result of the action of fire, which may have been used in funeral rites, or may have been accidental; very rarely can it have been produced by the reactions mentioned above, as has been suggested by Stapff.
Iron which has been in contact with the bone ash of burnt corpses has certain characteristics. When entirely surrounded with bone ash objects are well preserved[25], and only covered with a thin layer of oxide. How far the ash has acted as a preservative, I will not hazard an opinion, having seen but few specimens, and these had been already varnished to preserve them.
Under certain conditions the phosphoric acid of the bones forms a thin bluish layer of iron phosphate, corresponding in composition to vivianite (Fe3P2O8.8H2O), as was pointed out by Jacobi in a series of objects in the Saalburg Museum at Homburg. These objects also are quite durable.
In earth so full of sodium chloride as is that of Egypt, objects of iron will be readily corroded, and the explanation given above will account for the paucity of iron remains of Egyptian origin. It is difficult, however, to find a satisfactory explanation for the fact that objects found in sea-water are specially well preserved. It may be that, in spite of the presence of free oxygen in solution in the water their complete [15] insulation from the atmospheric air has resulted in the preservation of the objects, as is the case with those which have lain in a stream of fresh water.
Copper and its alloys are subject to the same far-reaching changes as iron, but the action is less rapid. Bronzes of widely different composition have to be dealt with to ensure their preservation, and to a less extent, copper also[26]. According to von Fellenberg[27] bronze objects may be classified according to the material in which they have been found, i.e. peat mud, water, or earth.
“(1) Bronzes from peat mud are covered with a black earthy mass, which can be easily removed by water and brushes, the alloy then assumes its metallic lustre and the characteristic colour of bronze. The complete preservation of the pure metallic surface of the bronzes, in the same condition as they were when they were submerged, is easily accounted for by the enclosure of the metal in mud of organic origin under several feet of water which effectually excludes the oxygen of the air.
(2) The bronzes found in water, as for example in the beds of lakes and rivers, are less perfectly preserved. They have usually a thin coating of a calcareous deposit, which however often allows the lustre and colour of the metal to appear in places. When such bronzes have dark or green coloured patches or spots, the layer is very thin and may be removed by treatment with acids, which allows the metallic colour to become visible. Bronzes [16] preserved in water still retain the same definite edges and points which they possessed when they entered the water. If bronzes which are markedly incrusted with verdigris are found in water in all probability they had lain in the ground a considerable time before being covered with water, and oxidation had penetrated deeply into the metal before immersion.
(3) Bronzes found in the earth or in graves appear covered with a fine green crust of verdigris which may be either light or dark in colour and which often has a vitreous lustre. This is generally known as Patina.
This crust varies in thickness from that of writing-paper to several millimetres. If the green crust be filed away, or better, removed by dilute nitric or sulphuric acid, the bronze is found to possess a reddish colour; below the crust of cupric carbonate is found a layer of cuprous oxide, which may be removed by ammonia, thus revealing the metal with its characteristic colour and lustre. This condition is characteristic of the slow oxidation of bronze in moist earth. The layer of cuprous oxide between the pure metal and the external crust of copper carbonate has been shown by the examination made by Dr Wibel to be a product of the reduction of copper carbonate by the metallic copper of the bronze. Bronzes belonging to this category have often lost their former metallic properties, and if of small diameter have often been completely converted into cuprous oxide, surrounded by a lustrous green or blue crust of carbonates. If a metallic core remains, it is found to be crystalline, brittle, and non-coherent, that is, it flies to pieces under the blow of a hammer. Fine ornamentation and sharpness, whether of edge or of point, have often disappeared. This does not occur with bronzes preserved in water.”
[17] In another volume of the series[28] von Fellenberg states that basic copper chloride occurs as a constituent of patina.
A few lengthier quotations may be conveniently given here, in part verbatim, in part abstracted from literature which is not readily accessible.
Reuss[29] states that it has been hitherto generally assumed that copper is first converted into cuprous oxide which is then converted into a green hydrated oxy-carbonate which is separated from the metal by a thin layer of cuprous oxide. The specimens examined by him, however, showed no such dividing layer, the metal being either directly in contact with the malachite [30], or else separated from it by a black or bluish layer of cupric oxide. He further draws attention to the occurrence of irregular knobs two to three lines in height which consist, in part, of azurite[31]. Neither oxides of tin nor chlorine were found. The alteration of the bronze he explains by the prolonged oxidising action of water containing carbonic acid.
In an exhaustive memoir Wibel[32] describes the various kinds of patina as malachite, copper-oxychloride, and azurite, with admixtures of tin oxide, silver, iron oxide, lead chloride and copper chloride. He discusses also the occurrence of the cuprous oxide layer which is said to have been described by Sage as early as 1779. After detailing the observations of Davy, Hünefeld, and Picht, that the metallic copper exists partly in alloy and partly free as crystals in the layer of cuprous oxide, he continues as follows[33]:
[18] “The process of decomposition in bronzes has been regarded as a slow oxidation, in which cuprous oxide marks the first and incomplete stage, while the carbonates represent the later completed phase. The formation of both these substances was assumed to be due to moist oxidation, on bronzes as well as in those superpositions of copper, cuprite, and malachite, so frequently found in minerals. Indeed, no other process of formation of the carbonates is conceivable; moreover cupric oxide, if really present, would be naturally regarded as a product of oxidation. The other substances, such as tin oxide, which are occasionally found, would be produced in part by similar simple processes, in part by the simultaneous action of particular salts, the chlorine compounds, for instance, by the presence of water containing sodium chloride. Similarly the production of cuprous oxide was usually attributed to an incomplete oxidation of the copper, although it might very well be the result of an inverse process, viz. the reduction of pre-existing cupric oxide.”
From the following considerations Wibel thinks that he is justified in his assumption that the layer of cuprous oxide is the result of reduction. Firstly, by no means all bronzes which have been dug up, even though from the same excavation, show the layer of cuprous oxide. Secondly, the cuprous oxide layer is in the crystallized state. Thirdly, ‘all the facts of chemistry show that the formation of cuprous oxide can only take place by reduction, given the ordinary conditions of temperature and pressure.’ Finally, in addition to oxygen and carbonic acid, many salts, those of ammonia for example, occur in the spots where bronzes are found and favour the formation of copper salts. Wibel also quotes in support of his views the experiment of Bucholz[34], [19] that a strip of copper, the upper half of which is immersed in a layer of distilled water, and the lower half in a concentrated neutral solution of copper nitrate carefully poured beneath it, becomes coated with copper and cuprous oxide.
He continues:
“Bronze objects are attacked by waters which contain oxygen, carbonic acid and a greater or less percentage of salts. Such soluble salts as are formed are removed by solution, while the bronzes become covered, according to circumstances, with an insoluble layer either of carbonate or of oxide, whereby the form of the objects is preserved. The water then penetrates by capillary action through the porous coating into the interior, and attacks further portions of the metal, forming a layer of soluble cupric salt; a portion of which is able to pass by diffusion through the external layer. For the same reasons the liquid, bounded as it is on one side by the metal and on the other by the almost insoluble crust, shows varying degrees of concentration: thus all the conditions necessary for the Bucholz process are fulfilled. If the water is rich in salts, a concentrated copper solution is formed and even metallic copper may be deposited from it (i.e. the ‘copper crystals’ of bronzes); but if, as is usually the case, the water contains only small quantities of salt, cuprous oxide crystals only are formed. The fact that the process takes place chiefly in the pores made by the water itself is readily understood, because of the comparative quiescence of the liquid; and that it causes a marked progressive change in the object arises from the continual exchange of a portion of the copper solution already formed with fresh solvent from outside. Where the absence of carbonic acid or other circumstances hinder the formation of an almost insoluble crust, the reactions detailed above may, under favourable conditions, [20] take place directly upon the surface of the bronze; if, on the other hand, there is a too rapid change of liquid (as for example in very wet localities), the process may altogether fail to set in, since the necessary conditions of rest, etc. are wanting. Since the absence of the necessary conditions may arise from a number of purely accidental causes, it will be easily understood, that bronzes from one and the same grave may show the same percentage of carbonates, but very dissimilar percentages of cuprous oxide. In short all actually observed conditions in which bronzes are found are accounted for by the explanations given above.”
The following extract is taken from the section dealing with patina in Bibra’s “Bronzes and Copper Alloys[35]”:
“The conditions under which Patina is formed, or rather the conditions under which copper alloys are gradually decomposed, are variable in the extreme. The four main factors which may be instrumental in determining the chemical changes may be thus stated:
(a) The composition (qualitative and quantitative) of the particular alloys.
(b) The mode of smelting and the original manipulation of the components, such as a good or poor mixing, fine or coarse grain, etc.
(c) The locality in which the alloy has lain.
(d) The length of time during which the alloy has been exposed to the particular conditions.... Marked differences may appear in the extent and nature of the chemical changes shown by the same alloy; thus one fragment while underground may have been enclosed in an urn containing bone ash and dry sand, while another fragment may have been in contact with decaying animal matter.”
[21] From what has been said above, the variations in the composition of patina may be readily explained. The composition has been found to be:
(α) Basic carbonate of copper.
(β) Basic carbonate and sulphide of copper.
(γ) Malachite (normal carbonate of copper), with occasional admixture of cuprous oxide and azurite (acid carbonate of copper) [Stolba].
(δ) Crystalline cuprous oxide, according to Wibel[36] a reduction product of the carbonate of copper, by the action of the copper of the bronze.
Lastly, copper chloride has been occasionally found in patina [Haidinger][37]. This is only to be expected from the varying character of the localities in which the statues or bronzes are found. The author has himself noticed on board ship, how objects of copper and brass, which are exposed to the salt spray, develop a durable coating of copper oxychloride [38] (atacamite).
In conclusion, reference may be made to a statement of Chevreul [39], who, after examination of both hollow and solid specimens of Egyptian statuettes, states that the bronze is of an excellent quality and that it occurs in four different conditions. He describes these four conditions, three of which are undoubtedly patina or altered copper, as follows:
(α) A green deposit with patches of blue.
(β) A blood-red mass.
(γ) A reddish coloured bronze.
(δ) Ordinary bronze unaltered in appearance.
The first in this category represents the ultimate stage [22] of decomposition of bronze and forms the outer incrustation of the statuettes. It is a compound of copper chloride and copper oxide and water in the same proportions as in Peruvian copper oxychloride (atacamite); the blue parts contain water, carbonic acid and cupric oxide. It is in fact the blue hydrated copper carbonate.
(β) The blood-red substance consists chiefly of cuprous oxide with an admixture of tin oxide. It contains chlorine, apparently as cuprous chloride, sometimes in considerable quantity.
(γ) The reddish colour seems to be due to the tin undergoing more alteration in the course of time than the copper.
(δ) The well-preserved bronzes are remarkable for the excellent quality of the alloy.
Chevreul continues:
“Copper and tin have thus undergone gradual changes from without inwards into chlorides, oxides and carbonates; the tin has been converted into oxide, the outermost layer of copper into oxide and chloride, while the layer in contact with the unaltered bronze beneath can only be oxidised into the suboxide.”
In a fissure in a statuette he found crystals of blue basic carbonate of copper, chloride of lead and hydrated oxychloride of copper.
Bibra himself examined the patina of several bronzes and found it to consist mainly of sulphate and carbonate of copper.
To complete the quotation from Chevreul’s work we may observe that he finds the cause of the formation of the patina to be the action of air, of water containing salt, and of carbonic acid. It is interesting that Chevreul succeeded in restoring a small bronze containing chlorine by reduction in a stream of hydrogen.
[23] In the year 1865 M. A. Terreil[40] published the analysis of a bronze patina containing chlorine. The result is as follows:
| Bronze. | Patina. | |
| Copper | 85·98 | 57·27 |
| Tin | 12·64 | 8·40 |
| Lead | 1·09 | 1·02 |
| Zinc | 0·50 | 0·46 |
| Iron | trace | 1·61 |
| Lime (CaO) | 0·13 | |
| Chlorine | 5·35 | |
| Carbonic acid (CO2) | 4·25 | |
| Alumina | 9·86 | |
| Water | 4·40 | |
| Oxygen | 7·25 | |
| 100·21 | 100·00 |
So too at a meeting of the Association for the Promotion of Industries in Prussia, Elster[41] referred to the existence of chlorine in patina, and regarded this as a proof that the patina upon antique bronzes was actually intentional on the part of the manufacturers.
E. Priwoznik [42] has described a rare kind of patina which formed a coating 5 to 7 mm. in thickness composed of three layers consisting of a reniform or botryoidal incrustation of an indigo blue colour. The uppermost layer which was also the thickest consisted of 33·22% of sulphur and 66·77% of copper, and was therefore cupric sulphide, CuS (which is known in the mineral world as Indigo Copper or Covelline). The second layer, which existed only in patches, was 0·5 mm. in thickness and of a blackish colour; it consisted of cuprous [24] sulphide, Cu2S with 15% of tin. The third layer which, like the second, was incomplete, formed a fine black powder, and consisted of 59·8 Cu2S, 23·2 Sn and 3·4% of water. The patina had been produced by the action of soluble sulphides or of sulphuretted hydrogen upon the copper, while the sulphur compounds themselves had resulted from the decay of organic matter in the soil in which the bronze was found.
Mitzopulos [43] described the green patina of the copper alloys found in Mycene as malachite and atacamite upon a reddish layer of cuprous oxide.
Another analysis of patina was made by J. Schuler[44]. The bronze in question had a grey outer layer, which passed gradually into a light green friable layer 2 mm. in thickness. A detached portion of this layer of patina, dried in a desiccator over concentrated sulphuric acid with a loss in weight of 9·44%, gave the following analysis:
| Tin oxide | 49·13% |
| Copper oxide | 22·46% |
| Lead oxide | 3·53% |
| Iron oxide and aluminium oxide | 1·75% |
| Silica and insoluble matter | 6·16% |
| Carbonic acid determined directly | 6·35% |
| Carbonic acid determined by ignition | 9·15% |
| Water determined by ignition | 14·43% |
Schuler calculates from these figures that the patina contains:
| 60·92% | H2SnO3 |
| 34·55% | CuCO3, CuH2O2 |
| 4·51% | (PbCO3)2PbH2O2. |
[25] The analysis of the bronze itself was as follows:
| Copper | 89·78% |
| Tin | 6·83% |
| Lead | 1·85% |
| Cobalt and Nickel | 0·90% |
| Iron | 0·28% |
Schuler makes the following observations:
“Whilst the percentage of copper in the alloy is high (89·78%) and the percentage of tin is low (6·83%), the percentage of copper in the patina (metallic copper 19·84%) is smaller, that of tin (metallic tin 42·67%) considerably greater. The percentage of lead in the patina has also slightly increased. One of the causes of this alteration in the proportion of the metals may lie in the fact that basic carbonate of copper is soluble in water containing free carbonic acid, whilst tin hydrate is insoluble. Another cause may be found in the action of water which contains in solution ammonia and ammonium carbonate produced by the decomposition of organic matter. Confirmative evidence of this supposition is the presence of small quantities of ammonia in the patina [45].”
Schliemann [46] asserts that bronze objects are destroyed by [26] copper chloride, and another reference to the presence of chlorine is made by Krause.[47]
Arche and Hassack[48] give the following details as the result of their analyses of three specimens of bronze:
| I. | II. | III. | |
| Copper | 66·97 | 73·40 | 71·98 |
| Lead | 17·27 | 14·77 | 18·37 |
| Tin | 11·98 | 5·09 | 7·20 |
| Antimony | 1·28 | 3·33 | |
| Arsenic | Trace | 0·82 | |
| Iron | 1·00 | 0·31 | 0·89 |
| Sulphur | 1·50 | 2·28 | 1·56 |
They obtain the following formulae and composition for the patina of the three bronzes[49]:
| I. | II. | III. | ||
| CuCO3, 2CuO2H2 | 85·83 | CuCO3, 3CuO2H2 | 95·11 | 56·08 |
| 2PbCO3, PbO2H2 | 13·01 | 4·49 | 24·62 | |
| SnO3H2 | 1·16 | 0·40 | 19·30 |
Reference may be here made to an article by Mond and Cuboni[50] published in the Report of the Academy of Florence, from which the following extract is taken:
“By the terms ‘rogna’ or ‘caries’ of bronze, archaeologists designate a peculiar change, to which ancient bronzes, as statues, coins, vases, etc. are sometimes liable when preserved in museums. This consists in a species [28] of efflorescence of light green colour at one or more points upon the surface, which spreads by degrees, like oil over a sheet of paper, destroying the surface and converting the interior of the bronze into an amorphous whitish-green powder. The rapidity with which this destruction proceeds varies much according to circumstances which are not yet sufficiently known. Sometimes the destructive spot grows so slowly that it is hardly perceptible even after some months; sometimes it grows very rapidly, numerous spots form, spread, and unite, until in a few months an ancient coin may be entirely destroyed. In this way antiquities which are valuable for their history, or for their workmanship, are sometimes more or less injured by this development of patina, which archaeologists regard as a plague in their collections.”
Mond and Cuboni believe that the growths above described are caused by Bacteria. Although they have not succeeded in producing the appearances of spreading patina by transference of cultures of bacteria to intact bronzes they think that their observations sufficiently support this supposition, which they believe is further strengthened by the fact that bronzes exposed for a quarter of an hour to a temperature of 300°F. (150°C.), whereby any bacteria would be killed, showed no further change after a period of six months. The following is an extract from an article by Berthelot[51]:
“Copper objects, which have been buried in the earth for several centuries, are found to be covered with a green patina and with an earthy layer of varying thickness which has the same colour. The metal itself is to a greater or less depth converted into cuprous oxide. [29] After removal the patina returns; in other words, the metal shows further growths, and when in contact with the atmosphere of our climate is in all cases by degrees converted into dust. These facts are well known to every collector and archaeologist, who designate the specimens thus affected ‘métaux malades’.... Analysis shows that the superficial green layer consists in great measure of atacamite (cuprous oxychloride) agreeing with the formula 3CuO, CuCl2, 4H2O. There are also found traces of sodium salts. The changes which have been observed are produced by salts from the soil, especially sodium chloride, held in solution by water. In fact a few drops of salt water placed upon a copper plate are sufficient for the formation of oxychloride.... This reaction is the result of the simultaneous action of the oxygen and of the carbonic acid of the air upon the copper and upon the sodium chloride in the presence of moisture, as is represented by the following equations:
4Cu + 4O = 4CuO
4CuO + 2NaCl + CO2 + 4H2O = 3CuO, CuCl2,
4H2O + Na2CO3.
Thus the continuous transposition which, under the influence of a salt-containing water, often acting in large volume, converts the metal into oxychloride, is readily explicable: while the process whereby the small quantity of sodium chloride originally present in an excavated bronze may cause its destruction after it has been placed in a museum is the following:
When the reactions given above have resulted in the formation of a certain amount of copper oxychloride, it is to be supposed that a small quantity of sodium chloride comes into simultaneous contact with the oxychloride and with the metallic copper. A slow reaction takes place and a double compound of cuprous chloride and sodium [30] chloride is formed. The remaining portion of copper is converted into cuprous oxide:
3CuO, CuCl2, 4H2O + 4Cu + 2NaCl = Cu2Cl2, 2NaCl + 3Cu2O + 4H2O.
The solution of the double salt is also in turn oxidized by the air which penetrates the whole mass. The result of the reaction is therefore sodium chloride, atacamite, and copper chloride:
3Cu2Cl2 + 3O + 4H2O = 3CuO, CuCl2, 4H2O + 2CuCl2.
The copper chloride which remains, if in contact with air and copper or even cuprous oxide, is similarly converted into oxychloride:
CuCl2 + 3Cu + 3O + 4H2O = 3CuO, CuCl2, 4H2O.
The cycle is thus complete, and its constant recurrence under the influence of oxygen and moisture is the cause of the destruction of those objects containing copper which are imbedded in earth, and even of those which are preserved in our museums.”
Finally a memoir by Villenoisy[52] should be noticed, the first portion of which is devoted to a proof that the patina of ancient bronzes is due to natural causes and is not the result of the art and methods of the metal-workers of the ancient world. The second portion deals with the various kinds of patina and their formation, as the following excerpts will show:
The following substances may be mentioned as capable of attacking alloys:—Ordinary oxygen, which has but a slight action on copper in the dry state but a more vigorous action in the presence of moisture, or as [31] ozone; sulphur also, ammonia, carbonic acid, and organic substances. Water has no direct influence, but acts as a solvent. The metals or metalloids of the alloys can unite independently with oxygen, sulphur, or carbonic acid, etc. to form oxides, sulphides, or carbonates; or again they can react among themselves and produce copper stannate or lead stannate. Ammonia will form ternary compounds or play a catalytic part. Whatever processes may result in the formation of patina, the changes which occur are too slow to allow their imitation and examination in the laboratory. The four metals which are found in ancient bronzes, viz. copper, tin, zinc, and lead, are particularly liable to certain changes. Copper forms chiefly cupric and cuprous oxides. The first of these is soluble in ammonia; the latter combines with ammonia to form a substance which is colourless, but which becomes blue on exposure to air. Tin forms stannic acid which probably produces stannates with copper and lead. Zinc becomes zinc oxide, lead is converted into oxides. Sulphur, as sulphuretted hydrogen, causes the formation of metallic sulphides. Ammonia has a threefold action, viz. it causes and furthers hydration, it is an energetic solvent, and it forms double salts. This last-mentioned action is particularly important in the formation of patina. Carbonic acid in the presence of moisture attacks copper, lead and iron, and, as a carbonate, exists in every metallic oxide which is exposed to the air. Several combinations of copper with carbonic acid are known, while lead is readily converted into lead carbonate by oxidation. The part played by the carbon compounds resulting from the decomposition of animal and vegetable substances has hitherto received little attention, but this decomposition of organic material is probably the chief cause of the beautiful blue patina. [32] The action of oxygen will depend upon the composition of the metal, upon the locality, and upon numerous other circumstances, while the colour of the patina will vary accordingly.
Villenoisy proposes to classify patina into three groups:
(1) Blue patina, with grey to blue-green and apple-green tints.
(2) Dark green patina.
(3) Black patina.
1. The blue patina produced by the action of ammonia upon the products of previous oxidation does not destroy the outer form of the bronzes, but is nevertheless unfavourable to the preservation of the metal, since the substratum of the patina is a porous mass, consisting of lead stannate and lead carbonate mixed with ammoniacal copper carbonate. The specimen has frequently an intact appearance, as if covered with a thin layer of oxide only, whilst in reality all traces of metal have already disappeared, and slight pressure often suffices to break the bronze into pieces. The nearer the colour of the patina approaches to grey, the less solid is the bronze likely to be, a result which is no doubt caused by the presence of lead carbonate. This type of patina has often a yellowish colour, especially on prominent parts, where, being porous, it has retained in its superficial layers substances which were in suspension in the subsoil water. The occurrence of a pale fine-grained patina of a uniform colour is in almost all cases due to the scaling off of patina belonging to this type.
2. Whilst blue patina is generally formed on bronzes which have been buried in earth, the dark green patina is formed both in the earth and also in the open air. The presence of lead seems to be an obstacle to its [33] formation. This dark green patina consists of variable proportions of basic copper hydrate and copper carbonate. The green layer frequently rests upon one of a red colour, a circumstance which proves that the dark green patina is almost always the result of two successive reactions: cuprous oxide is first formed and subsequently takes up water and carbonic acid. Tin is present as copper stannate. The cuprous oxide, which is generally regarded as unaffected by air, is perhaps drawn into further reaction through the agency of ammonia. In those situations where there is a flow of rain water a certain translucency of the green patina is often produced, and this is also possibly caused by ammonia. Unlike the blue patina, the dark green variety assists the preservation of bronze.
3. Black patina is probably due to a variety of circumstances. The substances which enter into its composition are cupric oxide, lead oxide, lead peroxide, copper sulphide and lead sulphide. If bronze does not contain lead it is blackened only by the action of sulphur. The rarity of black patina is no doubt due to the rapid oxidation of the copper on the originally rough, unpolished surface, which leads to the formation of a green patina.
These extracts show how little value can be attached to a classification of bronzes from the character of the patina present: the views upon the subject are so divergent, while the actual composition of the incrustations which form the patina and their external appearance are so widely different. In fact only two groups of bronzes may be distinguished, i.e. those which show patina and those from which patina is absent.
The first group comprises almost all the bronzes which are found in peat, which show, with rare exceptions, a metallic, often somewhat darkened, surface. Their state of preservation depends upon the nature of the peat in which they are found, but the metal surface has, in the majority of cases, become [34] somewhat rough and etched, although all the details are clearly distinguishable. More rarely one side retains the original polished surface while the other side is much corroded. If a much corroded bronze is found, the peat in which it has lain has probably contained free sulphuric acid (see also p. 13). All bronzes found in water must be included also in this group. The second group will then comprise all bronzes with an oxidized patina.
The classification given by Villenoisy seems entirely unsuitable, for it does not by any means exhaust all the kinds of patina which may occur. Thus no mention is made by him of the frequent occurrence of a patina which contains chlorine. If we separate the dark brown and the blackish patina, in so far as these two colours are pure, from those of a green colour, the first two varieties cannot be regarded as groups, because the tones of colour differ too much, and because, as Villenoisy himself observes, widely different patinas often occur on one and the same bronze. The durability of a patina upon a bronze cannot be judged either by the outer appearance or by the chemical composition alone. The fact that there has been no alteration in the outward appearance for many years offers no guarantee against further changes taking place. Thus a Minotaur [53] in the Berlin Museum, which for many years had shown no sign of change, was eventually found to be completely covered with numerous bright green spots over its entire surface. My own opinion is that the only patina which is really stable is that which consists of combinations of oxygen, hydrogen and carbonic acid with the metal, somewhat similar to those analysed by Schuler (see page 24), and by Arche and Hassack (see page 27). The presence of sulphides, and even of sulphates, does not seem to be injurious.
If a patina is to deserve the name of a good, sound, or, as it is termed, a “noble” patina (Edel-patina), the original [35] contours of the bronze with all its markings must be distinctly visible. For this the patina must not be too thick, must be of moderate hardness, and above all must have an enamel-like surface. Apart from chemical influences, such a patina can only have been formed in those cases in which the alloy has been homogeneous, fine-grained, dense and not porous, and when its surface has been so smooth that oxidation has taken place very slowly. Under these conditions the colour of the patina may vary greatly, for it may be [36] bright green, blue, or of darker shades from yellowish to brown, or even black. These latter tints often denote patina layers of very slight thickness. My own observations confirm Villenoisy's view that the brown and the black patina are for the most part due to the presence of lead in the bronze. Rein[54] holds the same opinion in regard to Japanese bronzes.
Certain forms of patina are not necessarily prejudicial to the preservation of bronzes, i.e. the green and blue varieties which have the composition of malachite (CuCO3, Cu(OH)2) and azurite (2CuCO3, Cu(OH)2), both of which are very often found on the same bronze. This variety of patina shows a crystalline structure. The simultaneous formation of both varieties, which is due to the greater exposure of one part of the bronze than another to the action of moisture, is well shown by a specimen in the Berlin Museum[55] (Fig. 6). This consists of the frontal portion of a Boeotian bridle, over parts of which leather straps had probably been tightly fixed. Those parts which had been thus somewhat protected from moisture were covered with blue azurite, which contains a smaller quantity of water. But the crystalline structure of these kinds of patina has often the disadvantage that the surface of the bronze is no longer clear, and consequently engraved markings and even stamped impressions are not visible. On page 142 may be seen illustrations of Roman coins, some parts of which are totally illegible. More frequently met with than these varieties or than the so-called “noble” patina, is that in which the bronze presents a more or less rough and pitted surface, light or dark green, or even grey in colour if there is a large proportion of lead present. More rarely the tint is blue or brown. The behaviour of such kinds of patina varies greatly, but durability is for the most part assured if, under the layer of green oxide, a reddish layer of [37] cuprous oxide is found. This rule is perhaps not invariable, for I have often found cuprous oxide present under the so-called spreading patina, but absent beneath one which is undoubtedly durable.
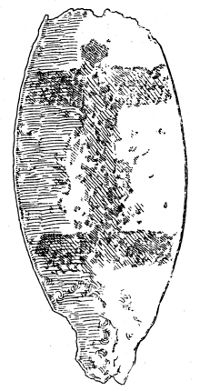
Fig. 6.
Portion of bronze horse-trappings showing blue and green patina.
Two instances may be here quoted as confirming Wibel’s view in reference to the reduction of cupric oxides to cuprous oxides and even to metallic copper (see page 17)[56]. In removing a sandy crust saturated with copper salts from a large Egyptian bronze[57], small crystalline masses of copper were seen here and there, separated from the metal beneath by a layer of cuprous oxide to which the admixture of tin gave a whitish tint. The copper was mostly deposited in slight depressions upon the surface of the metal and could be easily removed. Similarly, upon an Etruscan mirror exhibited in the Berlin Museum[58], reduced copper can still be seen forming red spots upon the lighter coloured surface of the bronze, which has already been freed from cupric oxide. The copper also can be removed with comparative ease, and is observed to be separated from the bronze by a thin whitish layer of tin oxide. A quantitative analysis of a small piece showed 100% of copper.
As has been remarked above, the layers of oxide frequently enclose grains of sand and even fragments of clay, earth, and ferruginous particles, so that the original contours of the bronzes are often indistinct or entirely obliterated (see Figures 41-43). These incrustations may occasionally be removed by a careful use of the hammer, but they are often so firmly united with the bronze, which is itself so oxidized, that removal by mechanical means is no longer possible.

Fig. 7.
Head of Osiris, showing advanced condition of warty patina[59].
These incrustations are however not so injurious as the tuberous and warty patina. Figure 8 shows an Etruscan mirror covered with a patina which generally results in the progressive destruction of the bronze[60].
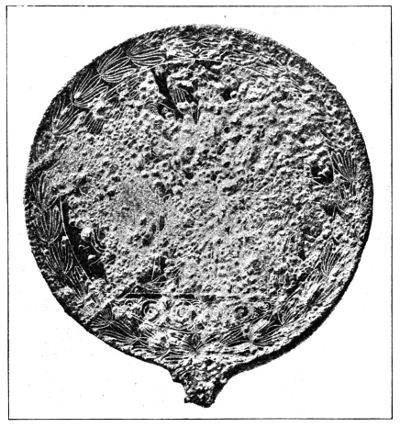
Fig. 8.
Etruscan mirror showing warty patina.
The following series of quantitative determinations of [39] chlorine obtained from the examination of bronzes in the Berlin Museums, shows conclusively the destructive influence of chlorine in the production of patina:
| Percentage of chlorine | ||
| Dark green “noble” patina (wine pitcher, Ant. Misc. Inv. 7161) | 0 | |
| Green patina on a layer of cuprous oxide (Etruscan vase, Ant. Fr. 1571) | 0 | |
| Dark blue “noble” patina (Etruscan wine pitcher, Ant. Fr. 608) | 0 | |
| Bright blue “noble” patina (Etruscan mirror, Ant. Misc. Inv. 7275) | 0 | |
| Bright blue “noble” patina (lid of vessel, Ant. Misc. Inv. 6322, 292 a) | 0 | |
| Hard greenish-yellow exfoliating patina upon a bright green, softer patina (Roman saucer, Ant. Fr. 1601 a) | 0 | |
| Bright green fairly firm patina, the colour rubbing off somewhat in parts (handle of vessel, Ant. Fr. 1440) | 0 | |
| A firm smooth green layer upon a brighter soft patina (mirror, Ant. Fr. 136) | 0 | |
| Blue crystalline patina (harness from Boeotia, Ant. Misc. Inv. 8579) | 0 | |
| Rough dark green patina (situla, Ant. Misc. Inv. 8509) | 0 | |
| Greenish “noble” patina (sword, Ant. Fr. 1144) | trace | |
| Rough green softer patina, with admixture of earth (funnel, Ant. Misc. Inv. 8582) | trace | |
| Dark green, compact warty patina (mirror, Ant. Fr. 32) | trace | |
| Green warty patina, with translucent cuprous oxide (mirror, Ant. Misc. Inv. 3312) | trace | |
| Green and blue crystalline patina (Buto, Aeg. 13135) | 1·7 | |
| Bright green cracked and warty patina (muzzle of the harness from Boeotia, Ant. Misc. Inv. 8579) (see Fig. 38) | 1·7 | |
| Green firm warty patina (Etruscan mirror, Ant. Fr. 53) | 2·1 | |
| Completely oxidized Cyprian bronze fragment (Ant.) | 2·2 | |
| Green cracked patina upon a thick layer of cuprous oxide (bronze fragment from Troy) | 4·0 | |
| Completely oxidized Cyprian bronze fragment (Ant.) | 4·2 | |
| Bright green efflorescent patches upon dark tuberous patina (bronze fragment, Ant.) | 5·9 | |
| Bright green powdery patina in the hollows of a darker smoother patina (Horus, Aeg. 11010) | 6·7 | |
| Bright blue powdery moist patina (Aeg. 12663) | 7·4 | |
| Green and blue patina mixed with grains of sand (Buto, Aeg. 13132) | 8·3 | |
| Bright green cracked patina (bronze fragment from Troy) | 9·3 | |
| Bright green powdery patches, dark green rough patina (cup, Ant. Fr. 1654) | 10·2 | [40] |
| Thick greenish black tuberous patina (Besa, Aeg. 9716) | 10·8 | |
| Green firm patina, with brighter patches (Buto, Aeg. 13787) | 11·3 | |
| Bright green powdery patina (Isis with Horus, Aeg. 14078) (copper) | 12·5 | |
| Green tuberous and cracked patina (Horus in the lotus flower, Aeg. 2409) | 13·1 | |
| Bright green powdery excrescences (Buto, Aeg. 13787) | 13·9 | |
| Bright green soft patina, with a dark and somewhat firmer surface (door hinge from Babylon, Aeg. V.A. 2185) | 15·1 |
[41] A due consideration of these figures must lead to the conclusion that as a rule a malignant patina is one which contains chlorine. That traces of chlorine are found in many cases of benign patina need cause no surprise, for frequent handling alone may suffice to bring about such a condition. Nor is this rule invalidated by the fact that a patina which is proved to contain chlorine (e.g. that of the mirror[61] depicted on page 40), has remained unchanged for years under certain conditions, for the formation of patina depends upon various causes, and it often happens that a bronze carries a patina which outwardly seems to have stood the test of years, yet internally oxidation has continued and becomes outwardly visible only when some mechanical injury to the patina allows variations of temperature to exert a greater influence. A specimen is often regarded as bronze, whereas in reality it does not even contain a metallic core, but consists merely of cuprous oxide, copper oxychloride, tin oxide, etc.[62], and is therefore incapable of further change. On the other hand it is not surprising to find a patina, which, although containing no chlorine, affords but a poor protection to the bronze, for in this case the cause may lie in the non-homogeneous and porous nature of the alloy.
This list shows in addition that this high chlorine-content is a distinguishing feature of the patina of Egyptian bronzes, as is only to be expected from the character of the Egyptian soil (vide pp. 1, 2 et seq.); in fact, although in most cases qualitatively only, I have proved the existence of chlorine in each Egyptian bronze without an exception. The destructive nature of chlorine is not often apparent in bronzes recently excavated, which usually show an apparently sound, dark [42] green patina with a smooth surface, sometimes like malachite or azurite; personally I have not met with any bronze object from Egypt which could be said to have a patina deserving the name of “noble” patina. Not till some time, or it may be not till years after the objects have been placed in [43] museums does the change become apparent, as has been so strikingly described by Mond and Cuboni (see page 27 ). The varying amount of moisture in our atmosphere undoubtedly influences the spread of the patina, which, if the application of a preservative is delayed, gradually eats into [44] the bronze. The adjoining figures (Fig. 9 to 12) of the same bronze before and after the process of preservation show distinctly such ravages, whereby the surface has been in some places eroded to a depth of 2 to 3 mm. In other cases, especially hollow bronzes, the thin walls have been completely [45] perforated. The explanation of these processes is found in the experimental work of Krefting[63], and also in the treatise by Berthelot, from which extracts have already been given. [46] The theory enunciated by Mond and Cuboni, that the “wild” or spreading patina is due to the action of bacteria, cannot now be maintained, for not only do chemical reactions give an adequate explanation of the process, but these observers have failed to transplant the bacteria; nor were the experiments of Dr Stavenhagen, undertaken at our request, more successful. That certain bacteria are capable of attacking metal, as for example the metal lettering on books, is an established fact, while the universal distribution of bacteria will naturally lead to their presence upon bronzes and their patina. The application of heat checks chemical change by driving off the moisture, and therefore arrests the spread of a patina for some time, until by penetrating the oxidized layer the moisture and carbonic acid can again act upon the patina and the underlying metal. As has been already stated in the passage from Dingler’s “Polytechnic Journal” quoted above, I have observed the renewed formation of efflorescence upon a bronze statuette which had been thus sterilised. This, it may be urged, was a case of re-infection: it is, however, strange that Mond and Cuboni do not refer to chlorine as a component of the patina. The presence of chlorine may have been overlooked; it cannot well have been absent, for in every case of rodent patina I have found without exception chlorine in the bright green efflorescences, whatever may have been the original source of the bronze.

Fig. 9.
Bronze Pasht showing destructive patina.

Fig. 10.
The same after treatment (Finkener’s method).

Fig. 11.
Bronze Pasht showing destructive patina.

Fig. 12.
The same after treatment (Finkener’s
method[64]).
Nor am I able to endorse the statement of Friedel[65] that a spreading patina is characterised by a peculiar and disagreeable smell, although some oxidized bronzes have a distinct smell which it is not easy to describe.
The presence of chlorine is particularly dangerous to those bronzes which consist of a casing of metal of variable thickness around a core of sandy clay, the object of which has been [47] to economize metal. These constitute an important class amongst Egyptian bronzes. The chlorine often exists in the core as sodium chloride, and can thus attack the metal from both sides. Moreover, the structure of many Egyptian statuettes of a later period is very porous and spongy, and thus presents a large surface to destructive agencies. On sawing through the support of an Osiris[66] numerous small bright spots were found, upon examination with a lens, to be small pores filled with a salt solution. A few days later the action of the carbonic acid had begun, and the bright spots of moisture were represented by small green patches. The following figures show the absorption of moisture and of carbonic acid by this specimen and by another Osiris from the Egyptian collection.
| I. Base of Osiris. | ||
|---|---|---|
| Weight, air-dried | 14·6554 gr. | |
| After one day in the desiccator | 14·6514 gr. | |
| Air-dried, after one day | 14·6540 gr. | |
| Air-dried, after ten days | 14·6576 gr. | |
| Air-dried, after one month | 14·6599 gr. | |
| Air-dried, after two months | 14·6623 gr. | |
| Air-dried, after four months | 14·7261 gr. | |
| After a prolonged period in the desiccator | 14·7033 gr. | |
| Air-dried, after one day | 14·7254 gr. | |
| Air-dried, after one month | 14·7321 gr. | |
| Air-dried, after two months | 14·7362 gr. | |
| Air-dried, after four months | 14·7381 gr. | |
| II. Osiris. | ||
| Weight, air-dried | 77·7522 gr. | |
| After some time in the desiccator | 77·7397 gr. | |
| Air-dried, after one day | 77·7462 gr. | |
| Air-dried, after ten days | 77·7548 gr. | |
| Air-dried, after one month | 77·7582 gr. | |
| Air-dried, after two months | 77·7617 gr. | |
| Air-dried, after four months | 77·7704 gr. | |
| After being heated to 200°C. in the drying stove and lying one day in the desiccator | 77·5967 gr. | [48] |
| Air-dried, after one day | 77·6752 gr. | |
| Air-dried, after one month | 77·8044 gr. | |
| Air-dried, after two months | 77·8191 gr. | |
| Air-dried, after four months | 77·8320 gr. | |
| Air-dried, after seven months | 77·8444 gr. | |
| After four days in the desiccator | 77·8225 gr. |
These figures show that in the first case the absorption of carbonic acid, oxygen, and water proceeded at first slowly, but more rapidly after three months, as was evidenced also by the appearance of marked efflorescence on the oxidized surfaces. The Osiris, which was more highly oxidized, showed a more rapid increase in weight from the first. The increased action after the heating was also manifest externally, for at the end of a fortnight the bright green efflorescences had made their appearance. In this case therefore the heating recommended by Mond and Cuboni, so far from proving beneficial, actually induced a more rapid decay.
The patina layer, as Schuler has also observed, often contains a greater proportion of tin than does the alloy; a result which is manifestly due to the solution and removal of the copper salts by the subsoil water. The bright efflorescences of an Egyptian statue of Buto[67] contained 10·49% of tin, while the percentage in the metal itself was only 7·66. In certain circumstances it may even result that an object which was originally composed of bronze is represented only by tin oxide [68]. The small proportion, and occasionally the complete absence, of copper is the result of the action of ammonia which may arise from the decomposition of dead bodies and of [49] carbonic acid, both of which agents, with the help of oxygen, attack the buried bronzes, and, dissolving the copper compounds by the subsoil water, leave only the insoluble tin oxide.
Upon the whole the foregoing remarks upon bronzes are equally applicable to objects of copper, which however appear to possess a greater power of resistance to the destructive action of carbonic acid and moisture, even where salt is present. This is probably due to the fact that the absence of tin and lead precludes any interaction between the compounds of these metals and those of copper. Copper objects with a sound so-called “noble” patina apparently do not occur.
Unless alloyed with a large amount of copper, in which case they show green efflorescences similar to those of bronzes, silver objects are almost always covered with a layer of soft silver chloride (horn-silver) of varying thickness, AgCl, or of the harder silver subchloride, Ag2Cl; and when these compounds form a thick layer, they often show a warty or more rarely a cracked surface. If the layer of chloride is thin, incised designs upon the silver will be visible both before and after removal of the chloride. The two chlorine compounds frequently appear together in distinct sharply defined layers of different colours, that nearer the silver being the layer of subchloride. This is especially well shown on fragments of silver from the Hildesheim silver-find[69]. Upon one fragment[70] the [50] layer of silver chloride was about twice as thick as that of the silver subchloride. Being unable to separate them I determined the silver and the chlorine of both layers together with the following result:
Silver 74·52. Chlorine 21·90.
Now for 2AgCl, Ag2Cl 74·52 silver would correspond to 18·11 chlorine only, while for AgCl the proportions would be 74·52 silver to 24·15 chlorine. Since the specific weight of silver subchloride is greater than that of silver chloride, these figures prove that the subchloride is also present.
Between the metal and the silver chloride there is often a thin powdery layer consisting of finely divided cupric oxide, or silver sulphide, and occasionally of gold, if, as is frequently the case, the silver is auriferous. The presence of gold may, however, also point to the existence of gilding. The silver chloride often shows a reddish or brown colour on the surface, due probably, in some cases, to the adherence of minute quantities of the earth in which it was found, but partly also to the action of light upon the silver chloride.
Thin black layers upon silver, as also the so-called silver tarnish, result from the formation of silver sulphide, from contact with decaying organic substances which have contained sulphur.
When placed in museums silver objects remain unaltered, and no further chemical changes take place.
Any other changes which have been observed will be gathered from the following extracts.
Church[71] analysed a specimen of silver upon which two layers were distinguishable. The outer semi-metallic layer consisted of metallic silver, with traces of chloride, sulphide, and iodide of silver, together with copper carbonate and a [51] small quantity of gold; the inner layer, which was soft, grey and powdery, had the following composition:
| Silver | 94·69% |
| Gold | 0·41% |
| Copper | 3·48% |
| Lead | 0·28% |
| Antimony with traces of arsenic and bismuth | 1·21% |
As the composition of the sound metallic core was identical, it is evident that physical and molecular changes only had taken place similar to those observed by Warrington[72] as early as 1843.
Silver objects found in Mycene are said by Mitzopulos[73] to show three layers, the outermost of which has a red colour and is not markedly friable, consisting of silver oxide; the second is tough and consists of silver chloride (horn-silver); while the third, that next to the metal, is similar to the outermost layer. Mitzopulos thinks that the chlorine must have been brought by rain water, since there are neither sea nor springs of water in the neighbourhood.
Schertel [74] distinguished two layers in fragments of silver from the Hildesheim silver find, the outermost of which proved to be silver chloride:
| Silver | 75·43% found, 75·31% calculated for AgCl |
| Chlorine | 24·51% found, 24·69% calculated for AgCl |
Beneath this layer was a very thin, almost black, brittle layer of silver subchloride:
| Silver | 87·0% found, 85·89% calculated |
| Chlorine | 12·8% found, 14·11% calculated |
Between the metal and the latter layer was a small [52] quantity of dark powder, which Schertel recognized as gold. He thinks that the layer of silver subchloride seems to indicate that the water, which permeated the surrounding clay, contained chlorides, and first converted the copper into copper chloride; that the copper chloride together with the silver then formed silver subchloride and cuprous chloride. Should the subchloride again become chloride, it would be able to attack the silver afresh. The slowness of the process, when the silver and copper in association with it had been converted into chlorine compounds, allowed the gold to be deposited as a fine powder upon the intact metal.
A silver coin rolled out into a thin plate, after remaining in a solution of common salt for six months, was found to have lost 27·7% of its copper, so that the plate became brittle, especially in those parts where it was thinnest.
Bibra[75] gives a similar explanation of the conversion into silver chloride. He believes that the reddish colour which is occasionally seen on silver at a fresh fracture must be due to the presence of cuprous oxide.
The following extract is taken from the section which deals with silver in the work of Berthelot[76] previously quoted:
“Silver chloride is for the most part produced by the sodium chloride dissolved in the subsoil water, which acts in conjunction with the oxygen and the carbonic acid of the air:
2Ag + O + (n + 2)NaCl + CO2 = 2AgCl, nNaCl + Na2CO3.
But this reaction differs from that which takes place in the case of copper in that it does not proceed continuously except in the presence of a considerable quantity of salt water only, as for instance in the sea. [53] In museums the alteration goes no further than corresponds to the minute quantity of sodium chloride contained in the object. On the other hand in an earth which contains salts, the continued presence of water can bring about a more or less marked change, and in some cases even a stable silver subchloride may be formed.”
Objects of lead have always a white appearance due to the formation of lead carbonate, as has been already mentioned above in connection with bronze. The carbonate is also often mixed with oxide.
Objects made of tin[77] are frequently found in pile-dwellings in a good state of preservation. They are, however, occasionally covered with white or brown layers of hydrated tin oxide, while in some cases oxidation has advanced so far that no trace of metallic tin is left in the hard grey masses of oxide which result.
Gold is found to be unaltered, or there is at most a thin layer of silver chloride, which is the result of the action of sodium chloride upon the silver which the gold usually contains. Gold objects often have a red coating, which has been found to consist of ferric oxide, and is due to extraneous deposits which have been fixed by the silver chloride. I have not been able to prove the presence of gold chloride[78], and it does not appear possible that water containing sodium chloride can have the power of acting upon gold. If the ferric oxide is removed mechanically, some of the gold will naturally be [54] removed with it, and this can be readily ascertained on analysis.
The degree of brittleness in objects of gold depends upon the changes which have taken place in other metals, especially silver, which are mixed with it.
Ancient glass, which is for the most part lime-soda silicate, exhibits a dull, rough surface with the well-known iridescence. The alkali is removed from the glass by the action of moisture, oxygen and carbonic acid, while the silicic acid remains in the form of minute scales, which cause the iridescence by interference. According to Bunsen the chemical action of the gases of the atmosphere on glass is facilitated by the condensation of water upon its surface; for the water thus condensed absorbs large quantities of carbonic acid. In certain circumstances almost the whole of the alkali is withdrawn from the glass. An analysis of glass of this kind, together with a discussion of the chemical reactions involved, is given in Muspratt’s “Chemistry[79].”
Glass objects which are markedly iridescent undergo gradual decay even under museum conditions; this is probably due to the continued action of carbonic acid.
The changes which organic substances undergo are various; thus, while leather becomes hard, papyrus becomes brittle. Like all other organic material they may undergo those destructive processes which are due to the growth of moulds or to the agency of various bacteria. They are also liable to be attacked by maggots, moths, and other insects. It is unnecessary here to describe in detail these numerous [55] and varied changes; a few special cases only need be mentioned.
Acid peat, in which iron objects perish, is found to have a good preservative action upon wool and horn, whilst vegetable fibres are destroyed. On the other hand, in pile-dwellings wool and horn substances have disappeared. Olshausen[80] thinks that animal fibre is destroyed by simple decay brought about by the oxygen in solution in ordinary water, whilst in peat the immense quantity of vegetable matter takes up the oxygen which can therefore no longer serve for the oxidation of wool and similar material.
Under certain circumstances woollen textures are found to be remarkably well preserved in oak coffins, as may be seen in the Museum at Copenhagen.
Bones, horn, and ivory show great variety in their behaviour, which depends of course on the nature of their surroundings. Thus for instance in acid peat sometimes the animal matter only is preserved[81], while in graves, beyond a few remains of tooth enamel, there is often nothing to show that they have enclosed bodies. Burned bones are generally found to resist decay, for the destruction of the animal matter leaves them no longer liable to further decomposition[82].
Amber objects are well preserved in water or in peat, but if they have lain in earth, they are darkened and often friable.
If organic substances, such as wood, etc., have lain in the immediate neighbourhood of oxidized bronze, and are thereby saturated with copper compounds, they show a very good state of preservation, which continues after they have been placed in a collection. Similarly the remains of fabrics upon iron [56] objects, which are permeated with rust, are sometimes found in good condition.
Objects imbedded in salt (sodium chloride) are in certain circumstances found in a good state of preservation and continue so, as is shown by the skins, leather and wooden articles which are exhibited in the Salzburg Museum.
As a general rule absence of moisture in the earth is essential for the preservation of organic substances, and is the cause of the splendid condition in which objects of organic material are found in Egypt. [57]
The object with which it is proposed to deal should first be photographed, and from different sides if necessary; for the external appearance is often changed during the process of preservation, and it is advisable that a representation of the specimen in its original condition should be kept in case any injury should befall the object, which however rarely happens if proper caution be observed. For this reason in the Laboratory of the Royal Museums at Berlin all bronzes are photographed before treatment, as also are all limestone blocks. Thus the 125 blocks from the Grave-chamber of Meten were each separately photographed. It is only in certain cases that this rule is not observed, as for instance in the case of the numerous Egyptian ostraca, i.e. fragments of earthenware showing inscriptions which had been previously copied.
The method formerly employed for the preservation of decaying and crumbling limestones was that of simple impregnation, and this is still followed in some cases which will be subsequently described. But as the active agents of destruction are not removed by this method the result is not always satisfactory, and an attempt is now made where [58] possible to remove those salts which are soluble in water, especially the sodium chloride, by the simple process of steeping in water.
If the presence of salt in a limestone is evidenced by a crumbling surface, or by the taste when touched with the tip of the tongue, the question will arise whether it will bear steeping, or whether the destruction is so far advanced that, on being placed in water, the limestone will fall to pieces.
If fractures or cracks can be actually seen in the stone, steeping is contra-indicated, but if the condition is less manifest, a preliminary test should be applied.
A large drop of water, e.g. about 25 cubic centimetre in volume, should be placed on the surface of the stone, and any changes which take place should be carefully noted. If the drop is not absorbed by the stone, it may be due to a layer of dust or to previous saturation with solutions of resin or varnish. Dust may be removed with a moderately hard brush or by rubbing with the finger, but if a limestone has been previously saturated with a varnish solution it will not absorb the water, and is therefore hardly suitable for this treatment. If the drop is absorbed, an iron or a steel point, such as the thick end of a medium-sized needle, should be used to ascertain whether the limestone at the moistened spot shows the same degree of hardness as elsewhere. If this is found to be the case, especially if the pieces are of a large size, the test should be repeated at other spots, including the back of the stone, for a hardened layer on the front aspect may be the result of former treatment. If the result of this examination is satisfactory and no soluble colouring is observed on the limestone, the process of steeping may be applied. If on the other hand the moistened area has become softer, or has become to any extent swollen, or if any colours which may be present show signs of disappearance or fading, treatment with water must be abandoned.
[59] The difference of behaviour is easily explained, for limestones do not always consist of lime only, or, more correctly, carbonate of lime (CaCO3), but often contain sand or clay, and the greater the amount of clay the more readily the stone softens or swells. Even when a limestone has borne this preliminary test satisfactorily it should be carefully watched for an hour or two after immersion and should be at once removed from the water should any further changes appear.
Steeping. The procedure to be observed is as follows. The rapidity with which the salts may be removed varies directly with the quantity of water used in steeping. The treatment of objects of small size presents no difficulties; any vessel of glass, porcelain, or earthenware will serve the purpose. Towards the end of the treatment distilled water should be used, or in default of this, clean rain water should be used in preference to that from a well. For larger objects (as, for example, the large limestone blocks of the Meten Chamber mentioned above, some of which were 1 metre in length and 1⁄2 metre or more in breadth and thickness), it is convenient to use wooden tubs fitted with a tap in front to draw off the water, and so tilted by means of stones placed underneath that the tap may be at the lowest point. The objects should not touch the bottom of the vessel. Smaller pieces may be suspended or may be made to rest on glass rings or supports of glass rods; while large objects should be laid on blocks of wood so placed as to allow the tub to be cleaned when necessary without removal of the blocks, the weight of which would otherwise entail much labour. The blocks should be as near to the surface of the water as possible, leaving a considerable depth of water beneath, for the heavier salt-laden water sinks to the bottom, thus bringing into contact with the limestone water with a smaller salt-content. The length of the steeping must depend upon the size and porosity of the limestone.
Under certain circumstances phenomena make their appearance [60] which must not be neglected. Thus if the treatment extends over a considerable length of time, the wooden tubs should be provided with lids to prevent the access of light. This was found indispensable in the treatment of the blocks from the Meten Chamber when Berlin tap-water[83] was used, for when the tubs were open a large quantity of brown hydrated ferric oxide appeared on the limestone, the roughness of which rendered its removal an impossibility even with brushes. This oxide is produced by various forms of algae and bacteria which developed in such numbers that the sides of the tubs were frequently covered with a layer of slime, which under the microscope appeared as a confused web of transparent threads[84]. This was brushed off with soft brushes at least once a fortnight, for the slimy covering impeded the access of the water to the limestone. That in this case the ferric oxide was the result of the action of light was proved by the fact that only those blocks which were placed near the windows were discoloured, and that the discolouration was proportionate to the amount of light which fell upon them[85]. Again, after the treatment in the covered tubs some of the blocks became so black that they resembled blocks of coal rather than limestone. After exposure to light for a day or two, especially when the water had been drawn off, the discolouration disappeared without leaving any traces. The colour was doubtless due to a minute quantity of iron in the form of sulphide which, after oxidation [61] in the air and light, became invisible upon the light yellow limestone. Under these circumstances the presence of sulphuretted hydrogen in the water, possibly produced by bacterial action upon the sulphates, was attested by the characteristic smell.
The enormous number of bacteria which develop in the water constitute a great hindrance to the process of steeping, and as to boil such a quantity of water as is required for these large objects is out of the question, frequent changes of water and frequent cleaning of the stone, wooden blocks, and tubs are the only remedies.
Examination of the Progress of the Steeping. The water should be changed at first daily, then by degrees every two, three, or four days, later on weekly only, until finally once a fortnight is sufficient. To ascertain the progress and completion of the elimination the quantity of chlorine in the wash-water may be determined by a simple method of titration [86].
The following short explanation may be of use, and the method is easily learnt. If a solution of silver nitrate is poured into a solution of common salt (sodium chloride), a white curdy precipitate is produced, a process which the following equation will explain:
NaCl + AgNO3 = AgCl + NaNO3.
The white precipitate is the silver chloride, whilst the [62] sodium nitrate which is produced at the same time remains in solution and is therefore not visible. As always definite proportions of the two substances, silver nitrate and sodium chloride, react upon one another, by the use of a solution containing a known amount of silver nitrate we can determine the amount of salt, and hence of chlorine present. By cautiously inclining a burette (Fig. 13) divided into tenths of cubic centimetres[87], the silver solution should be dropped into a beaker containing a definite volume of the solution to be examined for chlorine; the level of the silver solution in the burette should be read off before and after pouring out, and the number of cubic centimetres of the silver solution required to precipitate the chlorine will thus be known.

Fig. 13. Gay-Lussac’s Burette. 1⁄6 nat. size.
The process when carried out in this manner has one defect, for it is necessary to allow the precipitate to settle in order to see clearly whether an additional drop of the silver solution will produce further precipitation, or whether it will merely cloud the fluid; this defect can, however, be remedied by means of a so-called “indicator.” A few drops of a concentrated solution of neutral yellow potassium chromate should be added to the fluid to be examined, which is thereby coloured yellow; [63] the solution is then shaken or stirred with a glass rod, while the silver nitrate is dropped in. Every drop of the silver solution will cause a red precipitate, the colour of which however disappears on stirring so long as there is any chlorine present; only when the silver solution has precipitated all the chlorine does the red colour become permanent, and thus the change of colour of the whole fluid from yellow to red shows with exactness the complete precipitation of the chlorine. For practical purposes all that is required is the so-called decinormal silver solution, and from the number of cubic centimetres of this solution which are required to precipitate all the chlorine the total amount of chlorine present can be readily calculated.
In steeping smaller objects before examination the whole of the water should be well stirred with a glass rod or poured two or three times from one vessel into another: 100, 50 or 25 cubic centimetres are then poured into a graduated glass or drawn up into a pipette. The water should be drawn up by suction slightly above the level of the mark upon the stem of the pipette, the upper end of which is immediately closed with the thumb. By slightly raising the thumb the water is allowed to run off until its upper surface is exactly level with the mark. The amount taken is then placed into a beaker (for 100 cubic centimetres a beaker of 400 c.c. capacity should be used), and, after the addition of a few drops of a solution of potassium chromate, is examined by titration. In the [64] treatment of large objects, for which tubs are required, the necessary quantity of water may be drawn by means of a long pipette from the bottom of the tub, where the quantity of salt is always greatest, or through the tap at the bottom of the tub (as was done with the blocks from the Meten Chamber, in which case about 1 litre was drawn off into a glass out of which 100 c.c. were taken for titration). To obtain results which are comparable, care must be taken that the object is always as nearly as possible in the same quantity of water. After placing the larger blocks in the water, one examination should be made during the first few days, when the titration may require 20 c.c. or more of silver solution. There is no need to examine for chlorine while the water is being frequently changed: indeed, in order to economise the silver solution, this need not be begun until the second month, when the water is changed every fortnight.
As has been stated above, it is only necessary to read off the number of cubic centimetres of the solution used in the titration, for the decrease in these figures is a sufficient indication of the progress of the operation, while the diminution of the chlorine-content may be taken as an indication of the simultaneous removal of the sulphates[88]. In the treatment of small objects in distilled water, the process may be regarded as complete if the red colour is obtained on the addition of from one to two drops (i.e. about 1 ⁄10 to 1⁄ 5 cubic centimetre) of the silver solution. If, when tap-water is used, and is being changed at intervals of a fortnight or a month, the estimations give a constant result between 0·6 and 1·0, the treatment need not be carried further.
The accompanying table shows the figures obtained from three large blocks from the Meten Chamber. They represent the number of cubic centimetres of decinormal silver solution used for 100 c.c. of the water, which was changed every fortnight. [65] The first column on the left shows the dates upon which the stones were placed in the tubs:
| 3 Feb. 1890 |
7 Apr. | 4 May | 2 June | 25 Aug. | 22 Sep. | 4 Nov. | 1 Dec. | 12 Jan. 1891 |
| 3·0 | 2·5 | 2·3 | 1·4 | 1·0 | 0·9 | 0·8 | 0·8 | |
| 25 Apr. 1893 |
9 May | 18 July | 1 Aug. | 25 Nov. | 23 Dec. | 16 Feb. 1894 |
29 Aug. | 12 Sep. |
| 6·0 | 5·6 | 4·9 | 2·0 | 1·7 | 1·5 | 0·8 | 0·8 | |
| 29 July 1893 |
28 Oct. | 25 Nov. | 23 Dec. | 20 Jan. 1894 |
16 Feb. | 1 Aug. | 29 Aug. | 12 Sep. |
| 3·0 | 2·0 | 1·5 | 1·3 | 1·1 | 0·7 | 0·8 | 0·7 |
When repeated examinations gave a fairly constant result of 0·7-0·8 cubic centimetre the process was regarded as complete, for Berlin tap-water itself contains small quantities of chlorine compounds, 100 c.c. requiring from 0·4 to 0·6 c.c. decinormal silver solution. Before using the water from a well or from waterworks, it should be examined to ascertain the number of cubic centimetres of silver solution required to produce the red colouration. As the amount of chlorine compounds in the water may vary it is advisable to repeat the examination[89].
The following table shows the rapidity with which salts can be completely extracted from small pieces of limestone. [66] The limestones were placed in tap-water in three glass cylinders, each containing 2 litres; the amount of silver solution required for the water was 0·45 c.c. per 100 c.c.
| No. of days of soaking in water | 1 | 1 | 1 | 2 | 2 | 4 | ||
| 1 | Weight in grammes 107 | 3·4 c.c. | 1·4 | 1·1 | 0·7 | 0·5 | 0·5 | cubic centimetres of decinormal silver solution. |
| 2 | Weight in grammes 66 | 3·0 c.c. | 1·2 | 0·7 | 0·5 | — | 0·5 | cubic centimetres of decinormal silver solution. |
| 3 | Weight in grammes 47 | 3·6 c.c. | 0·8 | 0·6 | 0·5 | — | 0·5 | cubic centimetres of decinormal silver solution. |
These figures show the numbers of the c.c. of silver solution used for every 100 c.c. of the water, which was changed after 1, 2, etc. days as shown above. After 9 to 11 days therefore the stones could be declared free from salt.
If the accuracy of the titration method be considered unnecessary, either on account of the small number or size of the objects to be treated, or for reasons of expense (the outlay required is however very small), a solution of unknown strength may be used. A comparison between the degree of turbidity produced on mixing the silver nitrate solution with the tap-water, and that produced with the wash-water, will enable the progress of the operation to be gauged.
Advantages and Disadvantages. Although steeping removes the cause of decay, i.e. the salts contained in the limestone, and although permanence may be considered as certain, there are certainly some disadvantages connected with the process, especially when the pieces, on account of their size, must remain in the water for some length of time. Some large and very thick blocks from the Meten Chamber required to be soaked for more than a year.
The small quantity of carbonic acid which is always found [67] in water dissolves small quantities of calcium carbonate, thus the sharp contours of prominent parts may become somewhat rounded. Limestones which have developed fissures may, on immersion, lose small portions which might otherwise have remained attached, though probably for a while only. In such cases it must be carefully noted from which block, and from which part of it, the fragment has broken off, in order that it may be replaced[90].
Limestones which are much cracked, or which are likely to fall to pieces, should be wrapped round with gauze, or held together with twine, before they are put in the water.
In addition to the permanent preservation of the object some other smaller advantages of this method may be mentioned: for example, the layer of dust which is often present is removed and thus traces of colours may be brought out by the steeping which had been concealed by it. Thus certain remains of colour mentioned by Lepsius[91] as being still visible in his time upon some of the blocks from the Meten Chamber were no longer visible when we took them in hand. Moreover traces of green colouring which were visible after the treatment in the eyes of a few large figures in relief were probably evidence that colours had formerly been present.
Drying. When the steeping is finished the limestone is taken out to be dried. Small objects may be placed upon a glass ring, wooden tripod or some such appliance, which admits air on all sides, and may thus be dried by the air only. A piece of paper laid loosely over them will protect them from dust. In winter a hot stove, or similar source of heat, affords a satisfactory method of drying, but wet stones must not of course be placed directly upon the hot iron stove plate [68] lest spots of rust should be produced upon the stone. Large blocks are preferably dried in drying chambers in which in summer time a strong draught is obtained by opening windows on opposite sides, and which in winter are strongly heated and opened every now and then for a short time. The limestones should be laid upon wooden blocks to allow air to pass beneath them, while they must be guarded from dust both above and at the sides with sheets of paper. Several months are often required to dry large blocks completely.
Impregnation. When limestones have been completely dried, especially if they are soft, it is often advisable to impregnate them with one or other of the impregnation agents. To economize material, large objects may be painted over once or twice with a solution of the material chosen, but smaller objects should be immersed in the solution until air-bubbles are no longer formed. If there is a supply of tap-water with sufficiently good pressure, rapid and complete penetration by the fluid can be ensured by placing the object in a vessel containing the necessary fluid under a bell glass, the air from which is then exhausted by a water air-pump [92]. Figure 14 illustrates the application of such an air-pump fixed to the water-tap by means of an india-rubber tube which is firmly bound with wire. An india-rubber stopper perforated to admit a glass tube is fixed in the top of the bell glass, while the smooth ground edge and the thick ground glass plate upon which [69] it rests are smeared with grease or vaseline. The side tube of the air-pump is connected with the interior of the bell glass by an india-rubber tube which is sufficiently strong to resist the pressure of the outer air, and thus when the tap is [70] opened the pressure of the flow of water carries with it the air from the bell glass with which the pump is connected. If the water-tap is suddenly turned off when the air is exhausted the pressure of the outer air will force the water into the bell and cause it to mix with the solution of resin or varnish. To prevent this, a stop-cock or valve should be inserted, or the water-tap should not be turned off until the stopper of the bell-glass has been cautiously raised. A second glass tube provided with a stop-cock may be passed through the india-rubber cork and connected with a manometer to measure the progressive action of the pump (Figure 15). When air-bubbles cease to come from the object under treatment, the glass tap should be closed and the manometer removed, after which the glass tap should be again opened and the water-tap closed[93].
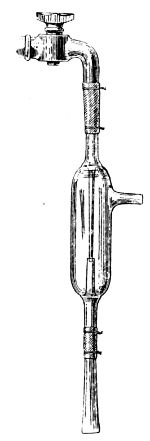
Fig. 14. Air-pump fixed to water-tap.
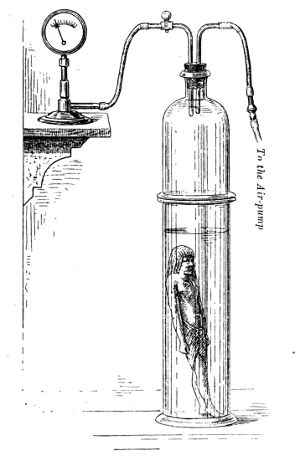
Fig. 15. Apparatus for impregnation by extraction of air fitted to manometer.
If the object is of some length but not too thick, the bell-glass may be fixed on a strong glass cylinder of a similar diameter having a ground edge (Fig. 15), into which the object and the impregnating solution are then placed.
The following solutions, amongst others, may be recommended as suitable for impregnation:
(1) Shellac dissolved in alcohol.
(2) Solution of gum-dammar[94].
15 grammes of dammar are dissolved in 130 grammes of benzine, to which is added a solution of 20 grammes of clarified poppy seed oil in turpentine. If the solution becomes too thick it should be diluted with benzine and a small quantity of turpentine.
[71] (3) Rice water or tapioca water[95].
(4) Dilute size.
(5) Waterglass solution.
(6) Linseed oil dissolved in benzine.
(7) Linseed varnish dissolved in 3 parts of benzine or petroleum ether.
(8) Solutions of stearine or paraffin wax in benzine.
(9) Collodion (free from acid). Zapon[96].
(10) Kessler’s fluate.
It may be added that, as a general rule, solutions for this purpose must be used as dilute as possible, for two immersions in a dilute solution are preferable to a single soaking in a concentrated one, which often scarcely penetrates into the pores.
As the preparation of solutions of shellac, gum-dammar, and of such substances as resin, stearine, and paraffin, necessitates heating, and as the solvents are very inflammable, it is advisable to make use of the solution of linseed varnish in benzine. This solution may be obtained at any time at any degree of concentration without the use of heat. Although it has the advantage that it hardens more rapidly than a simple solution of linseed oil it has also one disadvantage, for it gives a somewhat darker colour to light-coloured limestones. No more of the mixture of varnish and benzine should be prepared than is required for the impregnation, for this solution, on standing, throws down a gelatinous precipitate which is not re-dissolved even by heating. As this alteration is accelerated by the action of light, the mixture should always be kept in a dark place.
Collodion and zapon[96], on account of the expense, should only be used for small objects. After impregnation the objects [72] should be covered with glass jars, cardboard boxes, etc., to prevent the precipitation of moisture upon them, as the result of the rapid evaporation of such volatile substances as benzine and ether upon exposure to the open air.
Rice water, tapioca water, or size (the latter of no greater strength than 2%) are only applicable to specimens which are kept in dry rooms, for in damp rooms they readily become sticky, and are liable to be attacked by moulds. Waterglass solution, probably because it is generally applied in too concentrated a form, instead of penetrating the object has a tendency to form a pellicle, which readily strips off. Even dilute solutions, however, are said to be unsuitable, from the liability to the efflorescence of alkali salts.
In the case of marble objects and antique statues of porous limestone, showing colours which are still bright on excavation, but which would soon fade, Rhousopulos[97] recommends impregnation with a very dilute solution (1 in 1000) of waterglass to preserve the colour. The solution should be as neutral as possible: in any case not alkaline. This is several times sprayed upon the object, which is allowed to completely dry between each spraying.
A material which is suitable for large objects to which the solution can only be applied upon the surface is Kessler’s fluate[98], which is soluble in water, and which hardens the limestone without completely closing the pores. It offers the additional advantage that it is applicable to thick limestone blocks, the dryness of which is not certain. The solutions numbered 1-9 must only be used when the limestone is dry throughout its mass. The fluate to be used in any particular [73] instance must be decided from the nature of the case. Those most generally applicable are magnesium and zinc fluates and the so-called “double fluate.”
The stones from the Meten Chamber were hardened in the following manner: The limestone blocks were placed upright and the surface dusted by the air-current from a Dechend’s spray apparatus[99] which was then used to spray them repeatedly with a solution of “double fluate” of sp. gr. 1·16. Owing, however, to the injurious effect of the fine spray of the fluate upon the nose and lungs the stones were turned to a horizontal position, and a solution of fluate of sp. gr. 1·38 was applied by means of a large brush until the fluid was no longer absorbed. For the treatment of limestones on which there are remains of colours the use of a solution of shellac, gum-dammar, or collodion is recommended. Fluates should not be applied until their suitability for the particular purpose has been tested.
All specimens should be kept after impregnation in rooms which as far as possible are free from dust, for the dust which falls upon the surface will set in the varnish whilst it is hardening.
Impregnation without Previous Steeping. If a preliminary examination has shown that specimens of limestone will not bear steeping in water, recourse can be had to impregnation only. The treatment of such specimens must be thorough, for merely to paint the fluid upon the surface with a brush almost invariably proves a failure. Instead of penetrating the stone the impregnating medium forms a firm coating which is liable to be lifted, and in parts broken, by the crystallisation of salts, and thus allows the destructive processes to continue uninterrupted. Aqueous [74] solutions, e.g. size, cannot of course be applied, and as it is necessary to make a preliminary trial of a fluate spray, it is generally found preferable to make use of the varnish-benzine mixture. In spite of this, salts may still make their appearance in the form of a crystalline powdery layer on the surface, which can be wiped off with a wet sponge; any moisture must however be removed with a soft dry linen cloth.
Removal of Incrustations and Dust. Incrustations of earth, lime, or gypsum should be washed off with water or removed by mechanical means, such as gentle rubbing with the finger. The solvent action of acids upon limestone precludes their use for this purpose. Any dust which adheres can be removed by rubbing with stale bread-crumb.
It is usually only necessary to clean marble with a soft brush and warm water, with the addition perhaps of some good neutral soap. In rare cases the presence of sulphates may perhaps cause some friability. The crystalline structure of marble renders steeping futile, and accordingly impregnation is resorted to. The use of Kessler’s fluates may be recommended. Adherent pitch or resin is best removed by a mixture of alcohol and ether. Alabaster seems to remain permanently sound and may be cleaned in the same way.
Steeping. The same line of treatment should be followed as in the case of limestones. A preliminary examination should always be made to test the power of resistance in water, which is always satisfactory if the clay has been sufficiently baked.
In the case of coloured terra-cotta care should be taken to ascertain whether the colours are likely to suffer during steeping. There is no danger of injury if the steeping is not too prolonged; in fact, the removal of the dust during the [75] procedure often brings out the colours more clearly. If the Egyptian ostraca (clay fragments with black script) require to be washed they should be carefully watched in order to preserve the script, and therefore should be placed in the bath in such a way that the lettering is visible.
These fragments are usually curved and bear the script upon the convex side, care should therefore be taken that they are completely immersed, and that no large air-bubbles prevent the access of the water to any part of the under-surface. The writing is done with either lamp-black or more rarely some form of iron ink, and is retained mechanically by the porous character of the ostraca. In the latter case the characters may be enhanced by the application of a dilute solution of tannic acid, which sometimes proves useful also for limestone pieces.
If these fragments are sufficiently few in number to allow each to be put into a separate glass vessel, the washing out of the salts is completed so quickly that there need be little danger of obscuring the script. When large numbers were to be washed and when the script was already indistinct I have employed the following method: After examination as to their fitness for immersion the fragments are placed on a wooden grating in a tub, in which they remain for a couple of days, during which the water is renewed once. They are then taken out and allowed to dry. All those which still show the script distinctly are separated and their steeping is completed, but the remainder, having been completely dried, perhaps on the top of a warm stove, are brushed over once or twice with a dilute (1:6) mixture of varnish and benzine in such a way that the surface is only moistened, and when dry shows no gloss. The pieces thus superficially varnished are kept in a dry place for about two months, until the varnish is hardened; the process of washing out the salt is then begun again. The thin coat of varnish fixes the script without [76] interfering with the steeping. The varnish solution must be dilute, for a thick coating will partially peel off from the object in the course of the steeping, or will remain in the pores in the form of opaque particles, and thus render the script illegible.
The same difficulty which arose in the treatment of the Meten limestones was frequently met with in the treatment of these ostraca. Those which were of a dark brown colour especially, and to a less degree also the red and the yellow, were covered with a slimy growth of algae. As the script is easily destroyed no attempt should be made to remove these algae from the side which bears the script even with the softest brush, although they should from time to time during steeping be brushed from the underside. The inconvenience caused by algae is, however, less marked in the treatment of earthenware, the light and porous character of which renders prolonged steeping needless, nor is there the same necessity to continue the steeping for the purpose of chlorine estimation. The following results were obtained in the treatment of 13 fragments, the average thickness of which was 1 cm. [3⁄8th inch], with an average superficial area of 1⁄10 sq. metre [4 inches]. The tub in which they were steeped contained 85 litres [181⁄2 gallons] of water.
100 cubic centimetres of the tap-water used were found to require 0·5 c.c. of the silver solution, and on each occasion this quantity of the water was tested.
| Water changed after | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 4 | 5 days |
| 100 c.c. of the water used in steeping required | 3·0 | 1·3 | 1·0 | 0·8 | 0·9 | 0·7 | 0·7 | 0·6 | 0·6 c.c. of silver solution |
[77] The water was changed at first daily, then every two days, and so on: the steeping could therefore be regarded as complete at the end of a fortnight.
A small figure of earthenware, which weighed only 28·9 grammes, was steeped in 11⁄2 litres of distilled water, and gave the following result for every 100 c.c. used:
Water changed after 2 days required 3·6 c.c. silver solution.
Water changed after 3 days required 0·4 c.c. silver solution.
Water changed after 4 days required 0·0 c.c. silver solution.
The steeping was, therefore, in reality complete after five days, and, as the steeping water was thoroughly mixed before the withdrawal of the 100 c.c., the total quantity of sodium chloride contained in the figure can be calculated as follows:
For the 15th part (viz. 100 c.c.) of the water 3·6 + 0·4, i.e. 4·0 c.c., of decinormal silver solution were used, which is equivalent to 15 × 4·0, i.e. 60 c.c., of silver solution for the whole quantity. Now 1 c.c. of this decinormal solution corresponds to 0·00584 gramme of sodium chloride; the water therefore contained 60 × 0·00584 gr., or 0·35 gr. sodium chloride. Thus the figure contained altogether 11⁄5% of sodium chloride.
In addition to the chlorine compounds, there was also a considerable quantity of sulphates, the presence and disappearance of which were tested by adding to a few cubic centimetres of the water a dilute solution of barium nitrate or of barium chloride[100]. The soluble barium salts give with sulphates a white precipitate or cloudiness of insoluble [78] barium sulphate. If therefore on the addition of a solution of barium nitrate no cloudiness appears, even after some time, it may be concluded that sulphates are no longer present in the water. When the ostraca have been washed and dried, it is often possible to make the script more distinct by varnishing them over with a varnish-benzine mixture (1:6).
It is advisable to subject friable objects of earthenware to the process of impregnation (cp. the impregnation of unbaked clay, p. 81).
The Removal of Incrustations. Incrustations of earth or lime can be easily removed if the earthenware has been well baked, but trial must first be made with a drop of dilute hydrochloric acid, whether the earthenware itself is not attacked by the acid. The specimen is then placed upon a glass ring or suspended in water containing 2% of hydrochloric acid [101]. This mixture, which must be renewed every 24 hours, will remove incrustations which it would be difficult to remove by mechanical means, while crystals of gypsum of considerable size, which are often found on clay tablets of Assyrian origin, are easily dissolved in from two to four days.
Figures 16 to 21 represent two Assyrian tablets which have been cleaned by myself in this manner. It will be seen that the cuneiform characters, which before treatment were almost invisible, are now distinctly legible.

Fig. 16. and
Fig. 17.
Assyrian clay tablet with incrustations.
Before and after treatment.

Fig. 18.,
Fig. 19.,
Fig. 20. and
Fig. 21.
Assyrian clay tablet before and after treatment.
After this treatment with acidulated water the acid must itself be removed by careful washing in pure water. Here too a solution of silver nitrate will serve as a test, for, so long [79] as any chlorine, and therefore any hydrochloric acid, is present in the water, a white precipitate or cloudiness is produced.
The method of titration with yellow potassium chromate is not applicable here, for the free acid prevents the appearance of the red precipitate. The steeping must therefore be continued in distilled water until the addition of silver nitrate no longer produces any cloudiness.
Baked earthenware which shows colouring, or which has incised lines filled with substances containing lime, must not be steeped in acidulated water, nor will ostraca bearing inscriptions in iron ink stand this treatment; these are, however, fortunately rare: in fact amongst several thousand fragments few have shown incrustations of lime or gypsum. Should any such be found a cautious attempt should be made to remove the incrustations by some mechanical means. [80] Rhousopulos[102] carries out the cleaning of Lecythoi[103] and clay vases, which are painted in water-colours and which have a thin white incrustation, by dipping them into a 5% solution of pure hydrochloric acid. As soon as the colours show the [81] least sign of running, or if an efflorescence makes its appearance, the vase is immediately removed and allowed to dry. It is then dipped into distilled water and allowed to dry a second time. Impregnation is not necessary.
“If the treatment is otherwise successful, but an earthy layer remains upon the colour, the spots which are thus affected are lightly touched with the finger whilst the object is still in the liquid. Rubbing, or any sort of mechanical attack, is absolutely out of the question.”
This process evidently requires the greatest care and constant attention.
Impregnation. If upon examination it is found that a drop of water softens the clay, the same line of treatment must be followed as in the case of limestones which exhibit a similar condition (see p. 73), i.e. they must be subjected to the process of impregnation[104]. As the colour of the clay objects is yellow-brown or red-brown, the varnish benzine mixture will be the most suitable application for the purpose. A considerable number of sun-dried Assyrian clay tablets treated in this manner have given good results, and have undergone no change during the last five years, in fact they may now even be laid in water without crumbling.
In the case of slightly baked or unbaked Babylonian clay tablets the method formerly employed was merely to remove deposits of lime, clay, gypsum, etc., by lifting or scraping them away with pointed or wedge-shaped tools, for the soft clay would not stand treatment with water, still less with [84] 2% hydrochloric acid. The difficulty in avoiding damage to the clay surface, when removing the deposit, makes this method both tedious and risky. Warming to 200-300°C. in a drying oven, or in an iron box embedded in sand, seldom aids the removal of incrustations; moreover, this treatment has no hardening effect upon the clay, and thus does not facilitate the removal of the injurious salts by soaking. A further expedient therefore remains, that of heating the clay to higher temperature, whereby it is fully baked and rendered capable of resisting subsequent treatment with water or 2% hydrochloric acid. At the Royal Museum this firing is done in muffle furnaces[105], the smaller of which has a capacity of about one cubic foot, and is heated by six and twelve Bunsen burners. The temperature is regulated in the same way as in porcelain manufacture by the use of Seger’s cones[105], which are placed in the muffle, where they can be seen through the observation aperture. To avoid cracking the heating must be gradual, the gas-supply being very gradually increased. The firing must at first be adjusted to cone 022 [590°C.; Watkin, No. 1, 1094°F.]; the gas is then turned off and the furnace allowed to cool as slowly as possible. To effect this the damper is closed and all openings into the muffle are made up with fire clay. The clay tablet is removed when quite cold (usually in 18-24 hours), and, as a rule, much of the incrustation can then be removed by means of a soft brush. Should the removal prove difficult, and a preliminary trial have shown that it will bear the treatment, the removal of the deposits [85] will be assisted by soaking for two or three days in water. Should the tablet prove capable of bearing treatment with 2% hydrochloric acid it may remain in the acid for 12 to 18 hours. If necessary the acid may be renewed once; it must then be thoroughly removed by steeping in ordinary water and finally in distilled water, until the wash-water is free from chlorides. After steeping, the tablets will be found somewhat softened and occasionally coated with a slimy growth of algae, care must therefore be used in changing or taking them from the water. The best way to handle them is to place the fingers of the two hands under the tablet.

Fig. 22. Babylonian clay cone before treatment.

Fig. 23. Babylonian clay cone after treatment—firing, treatment with hydrochloric acid and steeping.
After thoroughly drying the tablets first in the air, then in the drying oven at a temperature of 212°F., supported on glass rings, it is well to impregnate them. This can be best carried out by placing them, while still warm, into melted paraffin wax, and raising the temperature to about 250°F. [120°C.]. The wax is allowed to cool to about 160°F. [70°C.], when the tablet is removed upon a broad band of gauze, any excess of wax is drained off, and the object is wiped with a soft cloth. The benzine-varnish mixture or zapon may also be used for impregnation. If heating in the muffle to Seger cone 022 is insufficient to allow of the removal of the incrustations, or if the condition of the clay does not warrant soaking in water or acid, the object must be again placed in the muffle and fired to Seger cone 010 [950°C.; Watkin, No. 13, 1742°F.], and, if softening occurs upon the application of water or acid after exposure to this temperature, recourse must be had to a third heating to Seger cone 05 [1050°C.; Watkin, No. 18, 1922°F.]. Higher temperatures than this are not advisable, for the lime, sodium chloride, and other salts found in some Babylonian tablets may partially fuse. During firing therefore the appearance of the object must be carefully watched, and the temperature lowered at once by reducing the gas-supply, if signs of fusion are noticed. [86]
Additional Methods of Impregnation. If clay objects have a smooth surface, it is, according to the “Merkbuch[106],” advisable to impregnate them with Belmontyl oil[107], for varnish in the course of drying gives a lacquered appearance to the surface. According to the same authority the surface of glazed vessels can be restored by impregnating them several times with a mixture of poppy seed oil and benzine [20 grammes clarified poppy seed oil in 270 gr. benzine, i.e. 1 in 131⁄2], and by subsequently brushing them first with soft, then with harder brushes. There are, however, many other substances used in different collections for impregnation, a few of which are subjoined.
In the Museum at Vienna friable clay objects are laid for two or three minutes in a dilute solution of warm size, and when dry are brushed over with a solution of shellac; size alone, or a solution of shellac alone, is frequently used for impregnation, or to give a coating. In the Museum at Wiesbaden thin specimens are impregnated with a solution of white of egg, brittle objects with dilute fish glue, while for hard objects a solution of shellac or melted shellac is used.
I have been able to wash out the sulphates from several Egyptian fayence figures in spite of the glaze, the fissures in which allowed the water to penetrate into the interior. The process of steeping, which was necessarily somewhat prolonged, was tested from time to time by the barium nitrate test (vide p. 77). [87]
These are rare, and in almost all cases contain salts. As, however, they will not bear steeping, they must be preserved by means of impregnation only. The varnish-benzine mixture should be used for this purpose.
These scarcely need any special preservative process, but Kessler’s fluates are useful for the impregnation of weathered sandstones which are exposed to the open air (see p. 72).
They can be cleaned by washing with warm water, while calcareous incrustations may be removed by hydrochloric acid. A thick coating of oil paint was successfully removed from an Egyptian statue of sandstone by placing it in an alcoholic solution of soda. Oil colours and similar substances may often be removed with ease and completeness from stone, plaster, wood, etc., by placing the objects in air-tight vessels together with a vessel containing alcohol. The alcohol vaporises, even at the ordinary room-temperature, and causes a softening of the paint. The time required for the treatment depends upon its age and hardness.
To fix together pieces of broken pottery good Cologne glue is useful, but it has the disadvantage that it can only be used when warm. For this reason it is better to use liquid fish-glue [Syndeticon], which may, if necessary, be thinned with a little vinegar. Fire-clay dust in waterglass is used in the [88] Museum at Breslau. A thick ropy solution of shellac[108] may also be mentioned, for the use of which the opposing surfaces must be first moistened with alcohol.
Gum arabic and dextrin should not be used, for objects thus cemented readily fall to pieces unless kept in perfectly dry rooms. This, however, may also be said of earthenware which contains salts, if cemented with glue or fish-glue. Previous steeping would obviate this difficulty.
Chalk, plaster of Paris, brick-dust, or fire-clay dust are often added to the fish-glue, dextrin, etc. Without giving additional strength to the cement, these substances may be of use in filling up small gaps between the fragments to be cemented.
For filling up larger gaps the “Merkbuch[109]” recommends stone cement, for the preparation of which it gives the following prescription:
“Mix 500 grammes of Cologne glue with three sheets of strong white blotting-paper, or four sheets of white tissue paper, shredded as small as possible, and boil until it becomes thick, stirring the whole into a perfectly smooth pulp. Let it boil thoroughly, and while stirring continually, and working with a stout wooden rod, add 21⁄2 kilogrammes of very finely sifted dry purified whiting. After working this mixture thoroughly, add 80 grammes of linseed oil, which must be also thoroughly worked in. To preserve the glue add 50 grammes of Venetian turpentine. This stone cement will take any shade of colour if mixed with lamp-black or coloured earths.”
The various methods for the preservation of iron objects which have been or are still in use may be divided into two groups. To the one group belong those methods in which the objects are preserved with their coating of rust, or with the rust that has penetrated them; to the other group belong those in which the removal of rust precedes preservation. The former methods must be applied when the iron has been completely converted into rust or when the rust has only left a small metallic core. These methods may of course be used also for all iron antiquities.
The methods of the second group can be applied to those objects only which still retain a strong metallic core, in which case the objects regain the more or less grey or white surface of fresh unoxidized iron. These methods are at present little known, and therefore but little used, for owners and the general public are still accustomed to see in the covering of rust the evidence of antiquity with which they are loth to part.
In addition to these methods, there are others which are of an intermediate kind, either special or a combination of methods from both these groups.
(1) Methods of preserving Objects of Iron without removal of the Rust.
Impregnation. The earliest processes, which are to some extent still in use in some collections, are simple impregnation methods, in which the object is either painted once or more with the impregnating medium by means of a brush, or is placed directly in the medium itself. In either case the penetrating power of the solution used is directly proportional to its fluidity.
[90] The following media may be used for the purpose:
(1) Warm size.
(2) Warm isinglass solution.
(3) Solution of waterglass.
(4) Solution of shellac in alcohol.
(5) Rubber solution in carbon bisulphide. The mass after swelling is dissolved in benzine[110].
(6) Copal varnish diluted with turpentine.
(7) Copal varnish mixed with linseed oil[111].
(8) Linseed oil.
(9) Linseed varnish.
(10) Linseed varnish mixed with an equal quantity of petroleum.
(11) Bees’-wax dissolved in turpentine.
(12) Bees’-wax dissolved in benzine.
(13) Petroleum.
(14) Vaseline.
(15) Melted paraffin.
(16) Oleate of lead: 100 grammes of olive oil, 100 gr. of lead oxide, and 100 gr. of water are boiled until all the water has evaporated and the mass has become grey. The mass is extracted by shaking it with alcohol, and the residue is dissolved in absolute ether, in the proportion of 100 gr. of ether to 5 gr. of the substance. Before use it should be diluted with a little ether[112].
[91] (17) Speerschneider’s mixture. This consists of 8 parts of rape oil, 1 part of bees’-wax, 1 part of pine resin, and 2 parts of benzene[113].
(18) Collodion, or the mixture used in the Museum at Donaueschingen, which consists of 30 grammes of collodion, 2 gr. of camphor, and 1 gr. of oxalic ether.
In addition to these materials, there are other mixtures of resin, varnishes, and bees’-wax, with their appropriate solvents, but they do not possess any special advantages as impregnating solutions.
After treatment with size or isinglass, iron objects may be given when dry a coating of linseed oil, linseed varnish, solution of shellac, etc.
The materials numbered 7 to 10 in the above list should be applied warm to enable the viscid fluids to penetrate the rust, for the more readily the solution enters the object the better is the result obtained. Apart from the fact that they are easily ignited at a high temperature, they must not be heated beyond 230°F. [110°C.], otherwise objects which consist largely of rust will fall to pieces[114].
In the process of impregnation a twofold result is aimed at, viz. to prevent the rust from crumbling, and to exclude air from the specimen. The application of heated linseed oil or linseed varnish is founded upon the supposition that these substances enter into a chemical combination with ferric oxide to form a stable compound; this is, however, disputed by some modern authorities[115]. Neutral substances offer a safer method for the exclusion of air, and of these melted paraffin is undoubtedly the best. The paraffin must be quite pure and [92] free from stearine, as can be ascertained from the melting point; thus pure paraffin melts at 130°-150°F. [55°-65°C.], stearine at 160°F. [70°C.]. Paraffin with a melting-point higher than 65°C. should be looked upon with suspicion.
In many collections the objects are heated before impregnation with media which are insoluble in water, or they are exposed to the air for six to twelve months after excavation. This latter proceeding is, however, certainly inadvisable if the iron contains chlorine, and if this is the case not one of these methods produces satisfactory results.
On the other hand, as almost all the iron antiquities which do not contain chlorine compounds may be treated by the methods of the second group, simple and direct impregnation is passing more and more out of use. Before impregnation all soluble substances, especially chlorine compounds, must be removed by steeping.
(2) Preservation by Steeping and Subsequent Impregnation.
Krause’s Method. The water used for steeping should be preferably lukewarm, and should be changed every twenty-four hours. It is even better, at least for the first time, to lay the object in water and then raise it to boiling-point, a measure which will allow the more ready penetration of the water. As in the case of limestones, earthenware, etc. (p. 59), care must be taken to place the objects as near to the surface of the water as is possible. Small objects may be put in glass jars, large ones in wooden troughs, tin vessels, or wooden boxes lined with zinc or lead. Any little excrescences on the iron, which are frequently filled with ferrous chloride, should be punctured to give the water unimpeded and more speedy access. Crumbling objects should be held together by tightly wrapping them in muslin. Curators must decide for [93] themselves how far means such as files, chisels, or small hammers may be used to remove the rust or earthy material conglomerated by rust.
Although much recommended, the method of adding soda or lime water to remove the chlorine as soluble sodium chloride or calcium chloride is, in our opinion, inadvisable. Both these substances precipitate the iron from the ferrous chloride or ferric chloride (which are soluble in water) as insoluble hydroxide of iron, which more or less closes the interstices, and thus impedes the access of water to the interior.
The process of steeping can here again be controlled by the use of the silver solution (p. 62), for if there no longer appears any or only very little cloudiness the steeping may be considered complete. The length of time required for steeping depends upon the thickness of the rust and the porosity or existence of cracks in it, and if the objects are of considerable size, it may extend over several weeks.
After steeping the object should either be dried in the open air, and later on a warm stove, or be placed for a few days in alcohol to remove the water, after which the rapid evaporation of the alcohol will quickly dry it. The steeping of iron objects in warm alcohol has been recommended[116], but if their size is considerable the method is an expensive one. This method has the advantage that the alcohol penetrates the rust sooner than does water, and also prevents oxidation, which may be actually produced by the water. It may perhaps be advisable to dilute the alcohol, the usual strength of which is 95% to 96%, with about an equal volume of water, for some salts are not readily soluble in pure alcohol. When dry the object is warmed for a few hours in a mixture of equal parts of good linseed varnish and petroleum. The petroleum serves to dilute the varnish, which can [94] thus more quickly permeate the entire mass of iron and rust. On account of the inflammable nature of the mixture the warming should be done over a water-bath. For small objects a cylinder made of ordinary tin-plate, measuring from 6 to 10 inches [15 to 25 cm.] in diameter and 6 in. [15 cm.] in height, may be used. To increase stability the lower half should be of a smaller diameter, and fitted into an iron tripod. The same end is attained by soldering a ring round the middle of the cylinder, which will rest on the ring of the tripod. The cover consists of a number of copper rings gradually diminishing in diameter, which fit closely into one another, thus enabling porcelain vessels of various sizes to be used. For larger objects, such as swords, two long rectangular troughs (Fig. 24) of stronger plate should be used. The following sizes will probably be found useful: one about 40 inches [100 cm.] long by 4 inches [10 cm.] broad, and 4 inches [10 cm.] deep, and the other slightly larger. Handles should be fixed at the upper edges. Three iron bars 1 inch [21⁄2 cm.] thick and 4 inches [10 cm.] in length are laid across the bottom of the larger trough, on which the smaller is placed. The space between the two vessels is filled with water to a depth of 2 inches [6 cm.]. The trough is warmed on a stove, or better, where gas can be had, by means of a number of [95] Bunsen burners fitted with rose or ring burners, over which the trough may be supported upon tripods. While heating care must be taken that the water does not boil over, which can be easily avoided by regulating the gas supply. As the water evaporates further quantities should be added as required. After simmering for about two hours, the objects should be removed and allowed to drain; they should then be placed on a tripod, or on glass rings, on the warm stove in cold weather, to accelerate the evaporation of the petroleum and the setting of the varnish. In summer drying chambers may be used; these are sold by dealers in physical and chemical apparatus, or can be made at little cost by a tinsmith.
Fig. 24. Water-bath. 1⁄15 nat. size.
If the objects have been steeped in pure alcohol, or at least towards the end of the treatment in three changes of alcohol, so that all the water is replaced by alcohol, they may be dipped directly without drying in the varnish mixture, for the alcohol evaporates in the varnish bath which is at a temperature of 194°-203°F. [90°-95°C.]. As the varnish hardens, the iron thus treated acquires a glazed surface; other means of impregnation may therefore appear preferable, e.g. a solution of gum-dammar or melted paraffin. For impregnation with the dammar solution the object must first be dried, and the air-pump used in the way described on p. 68. On account of the inflammable nature of the benzine heat must not be applied, nor indeed is it necessary.
When impregnating with pure paraffin[117], specimens may be lightly wiped with a cloth, but need not be dried. The paraffin may be heated to 212°-248°F. [100°-120°C.] without danger, so long as it is kept from direct contact with the flame. A thermometer should be used, and, as soon as the paraffin has melted at a temperature of about 60°C., the [96] object should be placed in it by means of tongs. When the temperature has risen above 212°F. [100°C.] the water is converted into steam, and causes a brisk ebullition of the melted paraffin. The quantity of paraffin used should, therefore, be such that its level remains at the least 2 inches [5 cm.] below the upper edge of the vessel.
When the bubbles have ceased to rise, thus showing that all the water is expelled, the paraffin should be allowed to cool to a temperature of 180°-190°F. [80°-90°C.]. The iron should be taken out with tongs, and the liquid allowed to run off. It should then be wrapped, while still at 80°C., in soft blotting-paper or in a piece of old linen to absorb the superfluous paraffin. If the surface of the object is very uneven, or if there are deep cracks or holes in which the paraffin can collect, it will, when cold, form a white mass, and should therefore, while still warm and fluid, be soaked up with filter paper, or distributed evenly by means of suitable brushes. The superfluous paraffin may also be absorbed by putting the object in dry sawdust; any sawdust which remains attached can be removed when cold with benzine, or it may be scraped from the spots where it has collected with a knife or spatula. Any spots where the iron may have become exposed may be covered with a thin coat of paraffin dissolved in benzine.
Ekhoff’s Method[118]. The objects are laid for two or three months in water which is changed every two or three days, a small quantity of quicklime being added[119]. After this steeping, and after some of the rust has been removed mechanically, the object is lightly dried and put into heavy petroleum of sp. gr. 0·85 to 0·95, which is then heated up to 220°F. [105°C.]. A thermometer should be used to ensure this temperature. This temperature being higher than the boiling-point of water, [97] the water contained in the object evaporates and causes the petroleum to bubble, as in the method previously described. When all the water has been replaced by the petroleum the bubbling ceases. After the fluid has somewhat cooled down, the iron is taken out and is allowed to remain for about an hour in sawdust, which absorbs the superfluous oil. Finally, while gently warming the object over a warm, but not too hot, stove, it is coated over with a mixture of 1 part of bees’-wax and 2 parts of turpentine, or better with paraffin dissolved in benzine. Heavy petroleum, which we have found by experience to be a suitable material, is preferable to varnish in so far as the iron is impregnated by a neutral substance which is practically liquid paraffin, but has the disadvantage of being highly inflammable and of being difficult to obtain at so high a specific gravity.
Straberger’s Method. This method, for the description of which I am indebted to Herr Straberger, has proved effective in the preservation of a number of iron antiquities in the Museum at Linz on the Danube. Even iron objects, which had been in bad condition and had undoubtedly contained chlorine, have after treatment by this method shown no signs of change, while the dull black surface has an agreeable appearance.
Straberger places the newly-excavated objects immediately into linseed oil to prevent the access of air. After remaining in the oil for some time they are taken out, wrapped in cloths saturated with linseed oil, and removed packed in sawdust. Upon arrival they are unwrapped and put into water, to which a small quantity of soda is added to remove the oil more easily. The water is frequently changed, and the objects are meanwhile cleaned mechanically with emery paper and hard brushes. Any blisters are removed by the aid of a small hammer and chisel. After steeping they are dried and smoked over a candle flame which is allowed to play over the whole [98] surface. The soot is then rubbed off with a cloth or soft brush. Objects with a smooth surface may be rubbed with india-rubber. The preservative action of this proceeding depends upon the fact that during the smoking, in addition to the soot, oily products of combustion are deposited from the candle flame, which prevent the access of air and moisture to the iron.
“Objects which are much decayed or cracked should, when cleaned and thoroughly dry, be again placed into linseed oil which has been slightly warmed and should remain therein for a few days before being smoked. Upon removal from this second oil bath they should be lightly wiped and dried over a moderately warm stove or in the sun. Patience is necessary, and nothing further should be done until the oil has entirely dried in the fine cracks and crevices and firmly binds the mass. The oil crust on the surface is then loosened by soaking in a strong soda solution and wiped off, after which the object is dried, smoked over the candle flame, and the soot wiped or brushed off with a soft brush. The smoking and wiping may be repeated if necessary.”
Herr Straberger states that his treatment has been successful when impregnation with isinglass and coating with shellac has failed.
The methods of Hartwich and Jacobi hold an intermediate place between the above methods and those which will be subsequently explained. With the former they have this in common that they do not call for the entire removal of the rust and that they require the use of linseed oil; on the other hand their application presupposes the existence of a strong metallic core, otherwise when the rust is removed they will show merely a skeleton of the original object. The existence of a sufficiently substantial metallic core can be easily ascertained [99] from the weight, for an object, which consists solely or in great part of the oxide of a metal, is much lighter than one of the same size which is largely metal. The ring also affords a test, for an iron object, of which the greatest part is metallic iron, gives a clearer note when struck than one which is chiefly rust. A still more certain test is the use of a file or a drill (comp. page 107).
Hartwich’s Method[120]. This method is intended for objects of an especially large size, the hard oxide coating of which does not allow satisfactory steeping. Hartwich heats the object to redness, allows it to cool slowly, and then scrapes off the outer layer which has been rendered friable by this treatment. The subsequent procedure is that of Krause’s method, viz. warming in linseed varnish.
Jacobi’s Method. The method of preservation of iron antiquities used in the Saalburg Museum at Homburg is described by Jacobi as follows: The object is heated in the fire of a forge, which causes the chief part of the rust to flake off, while any rust which still adheres is removed when cold by water and brushing. The object is again held in the flame with tongs and heated (smaller objects may be placed on an iron plate); and during the heating is quickly taken out three or four times and each time brushed over with linseed oil. Most of the linseed oil is thus burnt and the deposition of carbon gives to the iron a black colour, while the oil which has been partially burnt or hardened by the heat produces a slight lustre. This process, as carried out at Homburg by a locksmith, is that which blacksmiths ordinarily use to blacken iron objects and to protect them from rust. The preservation has proved permanent, and only in rare cases has it been found necessary to repeat the process. These good results are probably due to the fact that the antiquities of iron preserved in that Museum are for the most part found in [100] good condition, having very little rust and certainly containing only a very small amount of chlorine. Iron articles which contain chlorine but which still have a good metal core, after washing, drying, and a cautious preliminary application of heat, are ready for treatment by Jacobi’s method.
Inlaid Iron Objects require especially cautious treatment. Although I have not had any personal experience in the treatment of objects of this kind, good results have been obtained in several Museums, especially in that at Mainz.
The following quotation from the “Merkbuch” (p. 75) describes the method which is applied at Mainz, where it probably originated:
“Objects of this kind which are likely to have been originally inlaid with silver, gold, copper or brass, as is frequently the case with objects of the Merovingian period, are not placed in alcohol after the steeping, but are warmed and dipped three or four times into a hot dilute solution of isinglass. The heating is necessary, otherwise the isinglass will set on the surface and will not penetrate into the interior. When the object has been dried and the isinglass has set, the layer of rust which covers the inlaid ornaments is scraped off with a graving tool, and any spongy hollow parts are filled up with a paste made of iron rust and isinglass, before the inlaid work is cleaned. During the scraping the object is held in the left hand on a little wooden board covered with plush or thick chamois leather, to which it is fixed as firmly as is necessary by means of a vice. In scraping special care must be taken that the graving tool follows the lines of the designs, for in scraping across the design it may slip under the flat silver thread and raise it out of its place. When the ornamentation has been completely laid bare, it is rubbed with emery cloth and then polished with a brush and fine emery powder. The [101] piece is then dipped into a solution of gum-dammar, and, when the surface is dry, emery is again used to remove the varnish, which gives the silver a slightly yellow colour. The object is then protected from the influence of air and moisture by the transparent retouching varnish of Sohnée frères (Paris).”
A modification of Krefting’s method (p. 108) has proved eminently successful in the treatment of iron objects inlaid with silver. Krause[121] recommends that the article be placed, with the inlaid surface downwards, for 24 hours in a mixture of
10 grammes of 40% acetic acid,
10 grammes of ammonium chloride,
70 grammes of distilled water,
10 grammes of aluminium powder.
It is then removed from the bath, carefully brushed and washed, and, if the inlaid work is not yet cleaned, is replaced in the bath. This is repeated until the inlaid work is completely exposed. Spots of ferroso-ferric oxide which are difficult to remove may be ground away by an emery wheel, care being taken that the inlaid surface is held against the lower side of the wheel (which must be rotated in the reverse direction) so that it is always in sight.
All the methods of this group, which have been applied to many articles in various Museums, exhibit one inherent defect, for any rust which remains after treatment may cause the continued oxidation of the iron. The effects of this action of rust are, I believe, extremely small, and it must at the same time be admitted that iron antiquities, even if they have been well steeped and afterwards impregnated, do not always remain in a permanent and sound state of preservation. If [102] in such a case the well-known small watery bubbles should make their appearance, the steeping has undoubtedly been insufficient. This evil can be remedied by gradually heating the object to redness to destroy the impregnating material, and by a careful repetition of the steeping and impregnation.
(3) Preservation of Iron Antiquities by Removal of the Rust.
Steffensen’s Method (Copenhagen). The objects are carefully heated over a flame and are then laid in dilute sulphuric acid. The sulphuric acid dissolves a certain amount of the iron, and it is found by experience that the chemical action is strongest at those spots where any rust remains, and that this is detached by the hydrogen which is produced. When the cleaning is sufficient, the iron is laid in a dilute soda solution to neutralise the acid, and is afterwards well washed with water and dried in an oven. When dry the iron is brushed over with a solution of bees’-wax (or better of paraffin) in benzine, the evaporation of which leaves a protective coating of bees’-wax or paraffin.
Blell’s Method. The method proposed by Blell and applied by him to many of the objects in his collection is distinct from that described above, although in its earlier stages the principle is the same. The following quotation is taken from the description of his method which the author read before the Antiquarian Society[122] at Königsberg:
“If a specimen is found to have a sufficiently strong core of iron it should be heated in the furnace to bright redness and then dipped into water. The expansion of the iron caused by the heat and the subsequent contraction caused by the sudden cooling thoroughly loosens the [103] layer of rust. Large iron objects with a strong and firmly attached incrustation of rust will require a repetition of the process. By this means not only is the rust converted into a red powder which is easily rubbed off, but the object itself is rendered more suitable for the subsequent treatment. At the same time the heating process removes any coating of oil, fat, etc., which may have remained from previous attempts at preservation, and which would interfere with the further stages of the process. Smaller or delicate specimens should be treated in the flame of a spirit-lamp, but special care must be taken that there is sufficient iron present. Sword blades and other tools and weapons with sharp edges should be heated only, for the sudden cooling may cause cracks in the cutting edges.”
To complete the removal of the incrustation of rust which has been loosened by the heating process, or by the heating and sudden cooling, the object should be placed
“in a well-stirred mixture composed of one part by weight of sulphuric acid in nine parts of water. Bubbles of hydrogen will immediately rise and the rust will begin to separate. In freshly prepared acid objects which are not very rusty will be freed from rust after four to six hours, those covered with a deeper layer of rust in about twelve hours, but several days, or even weeks, may be necessary. The duration of the process depends upon the strength of the acid and the character of the rust, viz. whether it is thick and solid, or thin and porous, and whether the iron is of a soft, or of a hard character.
When first making use of this method it is advisable to use dilute acid and to take out the objects several times in the course of the day and examine them, while [104] during the night they should be taken out of the acid and placed in soft water[123].
For the acid bath and for rinsing it will be found convenient to have two pairs of wooden troughs having the following internal measurements:
(1) An internal length of 10 inches [25 cm.] by 71⁄2 inches [19 cm.] in breadth and 43⁄4 inches [12 cm.] in depth, which will be useful for the larger number of objects.
(2) For long narrow objects, e.g. sword-blades, and long spear-heads, the internal measurements should be 40 inches [100 cm.] long by 4 inches [10 cm.] broad and 3 inches [8 cm.] deep.
Small fragile objects are most satisfactorily treated in glass vessels or glazed earthen pots or vases.
The acid must have free access to all parts of the object; if a sword, for example, lies flat upon the bottom, the under-surface apparently remains unacted upon by the acid. This should be remedied by the use of a couple of small wooden supports.
Frequent rubbing with a cloth and forge scale[124] or coarse sand greatly helps in removing the rust, but gentler treatment is required for the smaller and more fragile objects. The rust is often very firmly attached in some portions of the object, and in this case those areas which have been already freed from rust should be coated over with lard, which is free from salt, to protect them from further action of the acid, while the pockets of rust [105] are alternately treated with acid and graving tools. No particle of rust should be allowed to remain, for sooner or later it will begin to spread, whatever precautions may be taken.
The action of the acid becomes less effective if it has been used for several objects. A little fresh acid should then be added. The more active the sulphuric acid, the brighter will be the grey colour of the iron after the rust has been removed. If old acid has been used the iron will be of a dirty grey colour, and should then be placed into fresh acid for a short time until it assumes a clear light grey colour.
The third part of the process begins with the removal of the iron from the acid bath and has as its object the removal of every trace of the acid, otherwise the rust will very quickly return and cover the whole surface. The object is therefore immediately and repeatedly rinsed in soft water and carefully dried; the cheapest material for this purpose is cotton waste, but ordinary linen-cloth must be used for objects with jagged edges, for the threads will catch in the notches and hinder the drying. This should be done without delay, or a change of the colour from light grey to yellow will betoken a new formation of rust. Articles showing a very complicated construction, which are however rare from the Iron Age, should be packed in perfectly dry hot pinewood sawdust, while those which are still more difficult to dry, for example, coats of chain-mail, after thorough rinsing, should be immediately put into a pan with melted lard, free from salt, and boiled until the cessation of bubbling shows that all the water has been driven off by evaporation.
They are then rubbed dry or are laid in hot sawdust, after which they are brushed over with melted lard and [106] placed in this condition for at least half an hour in a moderately hot cupboard until the fat has penetrated into the finest pores of the iron. That this has really taken place may be proved by the use of a file.
When by this means all trace of sulphuric acid has been removed the fourth stage of the process is reached, viz. the removal of the grease from the surface and the subsequent application of some preparation to prevent the access of air and moisture. Most of the grease is removed by placing the objects in a warm place on blotting-paper. Any grease still remaining on the surface can be entirely removed with a cloth or paint-brush by means of benzine. If no restoration or repair is required nothing more is necessary than to apply the protecting solution.”
A white varnish has much to recommend it from its protective power, but as it gives to iron an unsatisfactory gloss, it is preferable to use a solution of bees’-wax in benzine.
Having made use of Blell’s method in a number of cases I have a few suggestions and modifications to offer. The heating should be carried out carefully and gradually, lest the sudden conversion of the moisture in the rust into steam should cause small explosions which would scatter pieces of rust. There is no danger of this if the objects are heated in an oven; they should not therefore be heated in an open flame. For smaller objects I use a box six inches [15 centimetres] square, of strong tin-plate loosely covered with an iron lid, or with a piece of asbestos sheet; but if the objects are large, e.g. swords, spearheads, etc., I heat them on a strong piece of tin-plate bent round to form a channel, and covered with a long piece of asbestos sheet, the edges of which are bent over the edges of the channel, to retain the heat as much as possible.
[107] It is advisable, in my experience, to use the sulphuric acid well diluted, e.g. in the proportion of 1 to 20, and to renew it several times if necessary. In mixing concentrated sulphuric acid with water great caution is required on account of the evolution of heat. The acid should be poured in a thin stream into the water, but not vice versâ, and the mixture should be constantly stirred with a glass rod. If a glass vessel is used for the mixing, it must not be too thick lest the heat should cause it to break, but the larger the proportion of water to the sulphuric acid, the less considerable will be the rise of temperature.
For boring out rust spots which have eaten deeply into the iron a dental drill can be used with success, and a great variety of drills and milling cutters can be obtained. The rinsing, which Blell carries out by moving the object to and fro close under the surface in a vessel full of water, may be sufficient for thin iron objects, such as swords, knives, spear-heads, and similar objects. Larger specimens should be freed from the acid by putting them into a still more dilute solution, and, when necessary, by steeping for a short time in water. It may also be advisable to put the objects into dilute soda solution to neutralize the sulphuric acid, but this does not do away with the necessity for steeping in water. The brown coating of rust which may possibly follow the steeping can be removed by the use of steel-wire brushes, which can now be made of such fine wire that their softness almost equals that of a moderately soft tooth-brush. Brass-wire brushes should not be used, on account of the yellow colour which they give to the iron. I always put the objects directly after steeping into clean fat heated to 250°F. [120°C.], for brushing over with fat and warming in a stove often caused a slight tarnish to cover the surface. I have also used paraffin wax instead of fat.
For the method of restoring iron antiquities and of filling [108] up large gaps, the reader should refer to Blell’s detailed account; it will here suffice to quote his statement that a mixture of iron filings with tin filings can be used for this purpose. These are melted and applied by the aid of a blowpipe.
The accompanying illustrations represent iron antiquities which have been treated by Blell’s method: the sword (Fig. 25) proved, after reduction, from its two ridges to be a scramasax; on the spear-head (Fig. 26) treatment revealed a small copper ring at the most constricted part, while the fibula, which previously had been a mass of rust, now shows the spiral which had been totally disguised.

Fig. 25. Iron sword treated by Blell’s method.

Fig. 26. Iron spear-head treated by Blell’s method.

Fig. 27. Iron fibula treated by Blell’s method.
Krefting’s Method. The electro-chemical method of Krefting was originally published in “Aarsberetning fra Foreningen till Norske Fortidsmindesmaerkers Bevaring,” 1892 (p. 51), but in “Finska Fornminnesföreningens Tidskrift[125]” there is a translation into German by H. Appelgren, and an additional series of observations and experiments by him. His remarks are equally applicable to Blell’s method, and the following extracts and quotations from this paper give Krefting’s method of procedure and the circumstances under which it should be applied.
[109] Small fragile objects such as fibulae, thin clasps and bracelets or those which are much eaten away by rust, are not suitable for this mode of treatment, thus:
“A knife which is much corroded, and which when taken out of the earth shows a distinctive form (for example, that of the Early Iron Age), may lose so much by the application of the electric current that every distinct sign of its original character is destroyed. The characteristic edges of a spear-head or of an axe of the late Iron Age, or the equally characteristic point of an [110] iron sword, may, if the rust has eaten deeply into them, be unrecognisable when removed from the electrolytic bath. A sword, the hilt of which is inlaid with copper wire or is plated with silver or gold, or the blade inlaid with inscriptions in gold, silver, or copper, may be totally destroyed by incautious treatment; for the ornamentation, if undermined by rust, may be detached with the rust from the underlying iron.”
On the other hand, objects of sound metallic iron covered with an incrustation of rust about 1⁄25 inch [1 millimètre] in thickness may be easily cleaned in this manner, but if on using a file the metal does not appear at all, or only at a depth of 1⁄8 inch [3 millimètres], great caution must be used. If there is reason to believe that there is gold or silver inlaid work undermined by rust, Appelgren recommends that the object should, as a preliminary, be laid in clean water, which should be renewed every day. After some time, three weeks at the most, sufficient rust will have been cleared away by carefully brushing with a steel brush to lay bare the ornamentation, at least in part, and it can then be ascertained whether there is any rust underneath which would, if Krefting’s method were used, cause the ornamentation to be detached.
The line of treatment is as follows: The metallic iron core is laid bare by filing in several places. The specimen is then wrapped with strips of zinc in such a way that the zinc is in actual contact with the bare metal (Fig. 28). The whole is then placed into a 5% solution of caustic soda[126]. [111] Appelgren uses a solution of 31⁄ 2-41⁄2 lbs. [11⁄2-2 kilogrammes] of caustic soda in 2 gallons [10 litres] of water. The rust is cleared away by voltaic action; the iron forms the negative pole, the zinc the positive of a voltaic cell, in which the water is resolved into its constituents, viz. oxygen and hydrogen. At the negative pole, i.e. the iron, the hydrogen rises up in small bubbles and acts in part by mechanically detaching the rust as in Blell’s method, in part also by the chemical conversion of the rust into metallic iron, or into a compound which contains a smaller quantity of oxygen than does ordinary rust. The oxygen combines with the zinc to form zinc oxide, which is dissolved in the soda solution. The process is usually completed in 24 hours [127]. The black powder which is loosely attached to the iron is best rubbed off with wet sand and fine wire brushes. Any hard pieces of black stable rust (Edelrost), magnetic oxide of iron, which have not yielded to the [112] electric current should be removed by means of a small chisel. After rinsing the object thoroughly in water, it should be [115] placed in melted paraffin at 240°F. [115°C.], which will expel every trace of moisture. On removal the melted paraffin should be allowed to drain off, and thus leave when cold a protective covering upon the iron[128].
The following points should be observed in the application of the method. Vessels of glass or glazed earthenware should be used for the reduction, while long swords can be put into tall glass cylinders or into wooden troughs, the interior of which must be coated over with paraffin. The soda solution must be kept in a closed glass bottle[129]. It should be diluted with water until the specific gravity, as shown by the hydrometer, is 1·06; the mixture will then contain about 5 per cent. of caustic soda. During the reduction process the mixture frequently assumes a brownish colour as the result of the presence of organic matter associated with the rust. On account of the dissolved zinc which it contains it cannot be used a second time, unless regenerated by boiling with quicklime. The solution is, however, so cheap that this is scarcely worth the trouble.
[116] The objects should be handled with metal tongs, and should not be touched with the hand until they have at least been dipped or rinsed in water, for the soda solution has an injurious effect upon the skin. A basin containing vinegar, dilute hydrochloric or sulphuric acid should always be at hand into which the fingers should be quickly dipped if they have been in contact with the caustic soda. These materials will serve also for cleaning the vessels used in the reduction process.
The zinc strips should be 1⁄4 to 1⁄3 inch [1⁄2 cm. to 1 cm.] in breadth, and should be cut out of a piece of sheet zinc of moderate thickness, but of sufficient pliability.
Any firmly fixed rust may be removed by mechanical means, such as the graver, drill, etc., as has been previously mentioned. If in rinsing a slight layer of oxide appears, although this is rare, it should be brushed off with a steel-wire brush.
If one portion only of a specimen requires reduction (the other portion having, for example, remains of wood attached, and therefore being unsuitable for reduction), that portion only should be wrapped with the zinc and immersed in the solution.
The results obtained by Krefting's preservation-process are [117] quite as surprising as those which are afforded by Blell’s method. Figure 29, taken from Appelgren’s work, shows the lower portion of a spear-head before and after treatment, by which it became apparent that the whole socket was plated with silver, with two engraved and gilded animal figures. Fig. 34 represents a piece of a sword, on which an inscription was brought to light by the reduction process.
Fig. 28. Krefting’s method. Iron spear-head wrapped with strips of zinc.
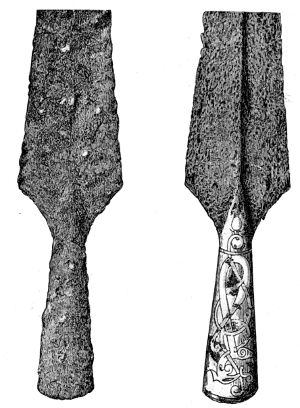
Fig. 29.
Iron spear-head before and after treatment by Krefting’s method.

Fig. 30. Iron pin from “Danes’ Graves,” Yorks. [Cp. Yorks. Phil. Soc. Report, 1897.]
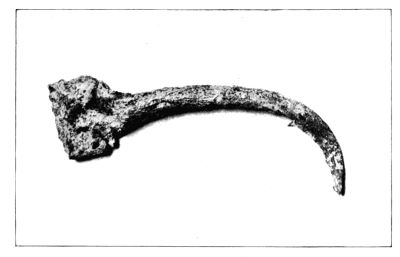
Fig. 31. The same after treatment by Krefting’s method, still showing chalky accretions.
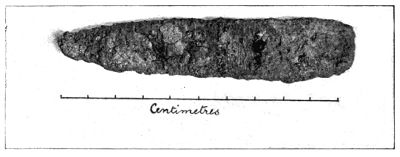
Fig. 32. Iron object from Lamel Hill[130], York. It appears to have been originally rivetted to wood or leather.

Fig. 33. After treatment by Krefting’s method.
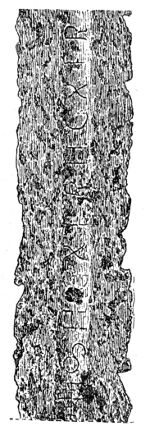
Fig. 34. Piece of iron sword-blade showing inscription, after treatment by Krefting’s method.
Hartwich’s Reduction Method[131]. This method is only applicable to small objects, because it necessitates the subjection of the objects to red-heat in a glass tube in a current of hydrogen. By these means the hydrogen combines with the oxygen of the oxides, which are thus reduced to metallic iron. Owing to the explosive nature of a mixture of hydrogen and air, this process should only be carried out by one who is conversant with chemical methods, for results which are equally good can be obtained at less expense by Krefting’s method. For Hartwich’s method a strong core of metal is essential, for although objects which are entirely oxidized may be thus reduced, the result will be the formation of a more or less loose iron powder which is frequently in [118] such a fine state of division that by union with the oxygen of the air, in consequence of the great amount of surface presented, it becomes red-hot with the formation of ferric oxide as a combustion product.
It is advisable to apply a combination of Blell’s or Krefting’s method with one of the first group (under certain conditions) to such iron objects as are found, during the process of preservation, to be penetrated by black stable rust to such a degree that the complete removal would only leave a kind of iron skeleton. Fig. 35 represents such an iron dagger-sheath [132], the dark spots upon it being rust. After heating and cooling down and a short treatment with acid the removal of the rust was proceeded with mechanically, but was not completed. The object was then well steeped, and when dry was warmed in the varnish-petroleum mixture[133].

Fig. 35. Iron dagger-sheath after treatment by a combination of Blell’s and Krefting’s methods.
Iron objects, the size of which is inconsiderable, such as arrow heads, small rings, etc., can be very quickly reduced, if they still have a well-preserved core, by heating them for a short time in molten potassium cyanide[134]. The cyanide may be melted in a porcelain crucible supported by wire gauze on a tripod over a good-sized Bunsen burner, and the object introduced by the aid of tongs. The reaction is accompanied by vigorous effervescence and is soon complete. It is then taken out and dropped into cold water. By repeatedly boiling in fresh quantities of water it is thoroughly cleansed, then treated with paraffin wax, or the water may be expelled by alcohol. It is then dried, and finally impregnated with zapon. If the cyanide treatment is insufficient, any remaining rust may be removed by drills or other suitable tools. Hitherto this method has only been applied to a small number of [119] objects, but there is no doubt that its use may be largely extended. Owing to the poisonous nature of the cyanide this method should be left to those who possess chemical knowledge. The disadvantage of the process lies in the difficulty of fusing large quantities of the potassium cyanide[135].
(4) Preservation of Medieval Iron Objects.
A complete treatise on this subject would be beyond the limits of a handbook, the following observations, therefore, will be sufficient for our purpose. The rust spots on objects of this kind are frequently only superficial and can be removed either mechanically by rubbing with pumice or emery, etc., or chemically by a concentrated solution of sodium sulphide[136]. To prepare this, sodium sulphide is dissolved in water, or flowers of sulphur are boiled in a solution of caustic soda. If the object is too large for immersion, the solution may be applied with a brush, and if the layer of rust is thick, the application must be repeated. After treatment the object must be rinsed in water and dried.
Small articles can be freed from rust by immersion in [120] strong fuming nitric acid[137], for strong acid dissolves the rust only, while it induces in the iron the so-called “passive[138]” condition in which it is not acted upon even by dilute acids, and can be safely washed in water. When thoroughly cleaned, the most suitable protective is some neutral substance such as paraffin wax, vaseline, or paraffin dissolved in benzine, but any of the numerous forms of oil or fat may be used.
Well-preserved bronzes with a stable patina, such as the highly esteemed glossy stable or “edel” patina, or that which, although not glossy, covers the bronze with a rough and often crystalline coating, should not be interfered with. Such bronzes as need treatment should be subjected either to simple cleaning or to some appropriate method of preservation.
The Cleaning of Bronzes. Bronzes, the metallic substance of which is more or less intact, while the surface is hidden under earthy or sandy material cemented together by copper compounds, may be cleaned either by mechanical or chemical means. When the materials forming the incrustation are more firmly cemented together than they are to the material beneath (which often still retains a polished surface), a small hammer may be used, but more adherent portions require the use of small chisels, which can be made to order in different shapes or sizes. I have used with advantage hammers with striking surfaces like those shown in Fig. 36. The two on the right are rounded so [121] that they touch the object at one point or on a line only. The process may be facilitated by the use of Springer’s method. A warm thick solution of glue should be spread upon the incrustation covering the bronze. As the glue dries and becomes cool it scales off, carrying with it some portion at least of the crust, thus leaving the metal clean. That part of the glue which remains can then be readily detached by gentle strokes with a hammer. The eyes should be protected when using the hammer, whether on the incrustation or on the glue.
Fig. 36. Hammer heads, natural size.
Other Methods. Since metallic oxides are scarcely, if at all, soluble in water, washing with water, even when a brush is used, will remove only earth or soil which is loosely attached. Compounds containing oxygen or oxygen and chlorine are, however, more or less soluble in ammonia, and, if they are thin and not too compact, after immersion for some time can be removed with a brush. Thick compact layers are loosened with difficulty.
Immersion in 2-5% hydrochloric acid acts more effectively, while sulphuric acid, nitric acid, and concentrated acetic acid have the same action. The frequent use of these reagents is, however, strongly to be deprecated, for it is impossible to remove the acid by simple washing with water after the incrustation has been removed. The bronze should be washed and placed in a very dilute soda solution or in dilute ammonia, after which it should be again well washed with distilled water. As has been explained in Part I., it is to chlorine compounds that the destruction of bronzes is chiefly due, and these are actually produced by the hydrochloric acid treatment. If the bronzes are not thoroughly washed, and this is no easy matter, sooner or later efflorescences will make their appearance, and the process of preservation must be repeated if the destructive action is to be arrested.
Various attempts have been made to remove the incrustation by raising the bronze to a red heat. This process is not [122] recommended; for not only does it give to the bronze an unpleasant appearance, but it detaches any inlaid metal (gold or silver) or enamel which may be present.
In conclusion, it may be stated that, although the process is slow and laborious, the best results are obtained by careful removal of incrustations by mechanical means.
Preservation of Bronze and Copper Objects.
(A.) Methods of Impregnation. The impregnation of bronzes, as of the majority of antiquities, has for some time been carried out by the use of solutions similar to those already enumerated for iron. These are applied directly or after the specimen has been either steeped in water or treated with dilute acids. This latter treatment, as has been already stated, is to be avoided, and if used all acid must be washed out before the object is dried. Steeping in water is of little use, because compounds containing oxygen or chlorine are often insoluble in water, which will at most only wash off loosely attached dirt or earthy material. The impregnation process may therefore be applied directly, and this should be done in all cases in which the surface is much corroded, warty (Figs. 7 and 8), or cracked (Figs. 37 and 38), or in which there is little or no core of metal. Impregnation is also the only means of preservation when the formation of oxides has raised inlaid metals or enamel in such a way that the removal of the oxides would detach them. The “Merkbuch[140]” recommends poppy seed oil and benzine mixture (p. 70) or the gum-dammar solution. To obtain thorough impregnation this should be carried out by extraction of the air, as has been already recommended in the case of limestone (p. 68). The object must also be perfectly dry, which may be insured either by exposure to moderate heat or by keeping it for [123] some time over anhydrous calcium chloride[141]. The object is placed under a glass bell jar, the edges of which are smeared with vaseline to ensure contact with the glass plate upon which it rests. The calcium chloride should be placed in an open glass vessel, beneath the bronze, but care must be taken that they are not in actual contact.

Fig. 37. Osiris showing cracking and destructive patina.
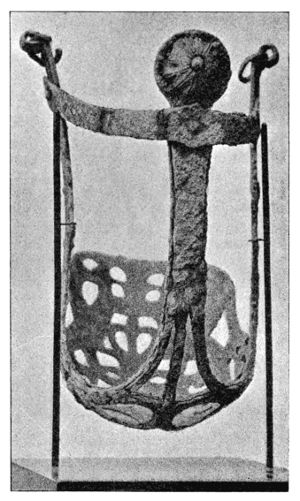
Fig. 38. Boeotian bridle with cracking patina.
[125] Immersion of bronzes in paraffin wax at 240°F. [115°-120°C.] gives results which are as good, if not better, than those obtained by the use of solutions.
Should efflorescences make their appearances upon bronzes which have been impregnated, their further spread may often be successfully prevented by smearing fish-glue on the parts affected. Fish-glue, however, has not proved a satisfactory material for the complete impregnation or coating of bronzes which are in the last stages of decay.
(B.) Preservation by Reduction. It has been previously explained (pp. 28 et seq.) that the efflorescences upon bronze known as creeping or malignant patina which may in time cause the complete destruction of the metal are due to the action of sodium chloride. It is found upon all Egyptian bronzes and upon those from some other localities.
The metal, especially the copper, is converted into the so-called basic chloride. In the reduction processes an attempt is made to reduce these compounds again to metal, while the chlorine thus liberated forms chemical compounds, which may be subsequently washed out with water. There are two methods which effect this reduction, viz., that of Finkener (Berlin) and that of Krefting. The principle of both is electrolytic, and both bring about the complete removal of the patina and the restoration of a clean metallic surface.
To complete this portion of the subject a third method may be mentioned, viz., reduction by heat in a stream of hydrogen. This method[142] is, however, only applicable to small objects.
Finkener’s Method. Care must be taken when examining the bronze that the metallic-looking mixture of cuprous oxide with other copper compounds is not mistaken for metallic copper. When it has been ascertained that the bronze still has a good metallic core, and that any inlaid [126] metals which may be present rest on the metal itself and not upon a crust of oxide, a platinum wire should be tightly wound round it. This should be connected by an insulated copper wire to the zinc or negative pole of the first of 3 or 4 Daniell cells, or, better, of two accumulators arranged in series. The object should then be immersed in a 2% aqueous solution of potassium cyanide. In the same solution, as near as possible to the bronze without actual contact, should be placed a piece of platinum foil connected first by an emerging platinum wire, and then by an insulated copper wire to the positive pole. The potassium cyanide completes the electric circuit and electrolysis takes place, whereby the water is split up into its constituents. The oxygen appears in small bubbles upon the platinum foil, but the hydrogen does not immediately make its appearance at the other pole, for, by combination with the chlorine and oxygen contained in the bronzes, free hydrochloric acid and water are formed. The hydrochloric acid in turn acts upon the potassium cyanide to form potassium chloride and hydrocyanic acid, both of which substances are dissolved in the water of the bath. The hydrocyanic acid can often be recognised in the room by its characteristic smell of bitter almonds. The process may be expressed by the following equations (neglecting the water produced by the oxygen of the oxide, which is of no importance in the process):
CuCl2 + 2H = Cu + 2HCl,
HCl + KCN = KCl + HCN.
Although the chief portion of the potassium chloride and hydrocyanic acid are dissolved in the bath, the remaining traces of these substances must be removed by very carefully washing the bronze in water, after which it should be dried, and if necessary finally subjected to impregnation.
[127] Some further observations may be made in connection with the practical application of this process.
Of course, other primary batteries may be used instead of the Daniell cells, but these latter may be specially recommended for the ease with which they can be procured and for the steadiness of their action. Information concerning the method of filling and using them may be obtained at any shop where they are sold. The copper wire and platinum wire should not be too thin, but must be at least from 1 to 2 mm. in thickness: they should be fastened together by binding-screws, and care must be taken that both the wire ends and the screws have clean surfaces. Glass vessels or glass cylinders are most suitable because the process of reduction can be watched, but large objects will of course require glazed earthenware baths. If wooden boxes are used they must be coated inside with paraffin wax. The strength of the cyanide solution should be 2%. Having a large number of reductions to carry out, I keep a 20% stock solution in a large bottle, one part of which is diluted with nine parts of water when required for use. Potassium cyanide is, as is well known, a strong poison, and care should therefore be taken to prevent access to any sore or cut on the hands; this can be done by the use of india-rubber finger stalls or gloves.
If the bronze object is neither too large nor too heavy it may be suspended in the bath by looping the platinum wire over the edge of the vessel. It is a convenient plan to use different coloured wires to distinguish the negative and positive poles of the battery, but should any doubt arise as to which wire should be connected with the bronze or which with the platinum, the following test will readily decide the question. Moisten a small piece of white filter paper with a drop of a solution of potassium iodide[143], and touch the two [128] conducting wires with it simultaneously: a brown spot will be seen on the paper at the point of contact with one of the wires; this is the positive wire, and must therefore be connected with the platinum. If the current is passing through the cyanide bath and the bronze, bubbles of gas will appear upon the platinum foil, or the products of the decomposition of the potassium cyanide may change the colour of the bath near the platinum to yellow or brown, while at the same time cloudy streaks under the bronze will show where the potassium chloride and hydrocyanic acid, resulting from the reduction of the copper compounds, are meeting with the cyanide of the bath. If the platinum wire is not firmly fixed round the bronze, hydrogen may be formed upon it, and should this occur the wire should be drawn tighter.
Whilst the reduction is going on it is advisable to renew the potassium cyanide at least once, or even several times, if large and greatly oxidized bronzes are under treatment, for otherwise all the potassium cyanide may be consumed by the changes in progress; this can be ascertained with certainty by a smell of chlorine. When the bath requires renewal the bronze may be taken out with a pair of metal tongs, or if too large, two strong copper wires should be passed underneath it, the ends of which are wound round a strong glass rod or wooden stick. The bronze should then be well rinsed or brushed with a soft brush before it is put into the fresh bath.
Bronzes are frequently met with which are much deformed by an earthy or sandy layer cemented by oxide. These incrustations can be partly removed by a preliminary treatment with dilute hydrochloric acid, but the bronze must be afterwards carefully rinsed with water or even steeped to prevent unnecessary decomposition of the cyanide by the acid. Before reduction it is useful to secure thorough penetration by placing the vessel containing the solution and the bronze [129] under a bell glass attached to an air pump, as has been previously explained (p. 68).
During the process of reduction small whitish-green crystalline needles often collect on the platinum foil, but although in large numbers they are so minute that it has not been possible hitherto to determine their composition; they seem to contain copper and cyanogen. After some time the platinum becomes covered with a whitish-green or brownish deposit, which should be removed by rinsing in water and brushing; if this should not succeed the platinum must be dipped in hydrochloric acid, rinsed with water, and rubbed with fine sand. The glass vessel may be cleaned in the same way.
The reduction is complete when all the chlorine, previously combined with the metal, has combined with the hydrogen produced by the electrolysis of the water. There being no further chlorine with which the hydrogen produced by the continued action of the current may unite, the completion of the process is marked by the appearance of bubbles of that gas upon the surface of the bronze. The bubbles which rise from beneath often mark out the outlines of the object upon the surface of the bath.
Before the bronze is washed it should be placed in a fresh cyanide bath, but of 1% strength only. For large and especially for thick objects, this bath must be renewed several times, so as to allow the washing process to begin in the bath itself whilst the current is still passing through it. Care should also be taken that every side of the object in turn faces the platinum foil for some time, for if one side remains turned toward the platinum throughout the process, it will sometimes assume the red tint of copper, while the rest of the bronze retains a somewhat dark colour.
When finally removed from the reducing bath, after the black metallic powder has been thoroughly cleaned off with [130] water and a soft brush, the object should be suspended for a short time in water at the ordinary temperature, or so fixed that there is a good depth of water beneath it; it should then be washed in hot water. When the bronze is first placed in water, whether hot or lukewarm, small bubbles of hydrogen will continue to rise for some time, while at the same time a whitish, or sometimes grey, gelatinous precipitate, consisting of a hydrated oxide of tin[144], will often fall from it. The grey colour is caused by the admixture of small particles of lead or copper.
At first I renew the water two or three times a day, then once in twenty-four hours, and finally at longer intervals, using distilled water throughout for small objects, but for larger specimens for the final washings only. For the earlier washings at any rate I use warm water. Cyanides as well as chlorides give a white precipitate with silver nitrate; this reagent will therefore serve to indicate the progress of the operation. If at the end of a fortnight in the case of small bronzes, or in three to six weeks for large objects, the water shows no cloudiness, or if upon the addition of yellow potassium chromate it instantly assumes a red colour (p. 62), the steeping may be considered complete. Some Egyptian bronzes, especially those which contain a large proportion of lead, after steeping exhibit a whitish crystalline coating of lead carbonate or small hemispherical groups of crystals scattered over the surface of the metal, especially where the pores are large; when dry these can easily be removed.
[131] An extended experience points to the conclusion that bronzes should be dried at once, and as quickly as possible. They should be wiped with soft cloths and then dried in a drying chamber or upon glass or metal rings on a stove. A simple form of drying chamber can be made with copper or iron plate of sufficient thickness, with a loose lid provided with a hole fitted with a cork, through which a thermometer passes. This can be heated over a Bunsen burner, but the temperature should not exceed 230°F. [110°C.]. Small objects may be freed from water by immersion in alcohol for twenty-four hours before drying.
The completion of the process may be gauged by the yellowish or reddish yellow colour which the bronzes should assume when they have been dried and wiped with a cloth or brushed; brushes made of the finest steel wire may be used for this purpose. A bright colour is but rarely seen on bronzes which contain lead. Egyptian bronzes frequently contain as much as 20% of lead, and such bronzes have nearly always a dull-grey or blackish appearance. A similar colour is seen on bronzes which contain no lead, but which are very porous, and are in an advanced state of decomposition. In such cases the finely divided particles of reduced metal are retained upon the rough surface of the bronze, and as all metals, when sufficiently finely divided, form a blackish powder without any metallic lustre, the whole object then appears almost black. It is difficult, and in many cases impossible, to remove this dust, especially that retained in the pores. Metal dust is injurious to the lungs, and if recourse is had to brushing, an efficient extractor for the removal of the dust-filled air is required[145]; but brushing and the use of bellows in addition frequently prove insufficient. Washing the objects [132] with benzine is more effectual, but a trustworthy method of giving the bronze a better appearance is to place it into melted paraffin wax[146] at 250°to 285°F. [120° to 140°C.]. Yet [133] the use of paraffin wax should be avoided if possible, for in spite of the most careful washing blue efflorescences may sometimes appear upon thick bronzes in the course of a year. If this should happen they must be washed out at once, and the bronze can again be submitted to the cyanide-reduction process. If however paraffin wax had been applied an attempt would have to be made to remove it by immersing the bronze in benzine or a mixture of ether and alcohol, or by heating, before the reduction process could be repeated.

Fig. 39. Bronze bull showing warty patina.

Fig. 40. The same after reduction by Finkener’s method[147].
[134] There is no doubt that these bright-blue efflorescences are the result of an incomplete reduction, which in many cases can scarcely be remedied, for it is often impossible thoroughly to wash objects of great thickness. Thin bronzes, bronze plate, and copper plate remain free from efflorescences. Moreover, many bronzes, especially Egyptian ones, have a hard, non-metallic core, which in the casting has been partly fused or at least hard-burnt, and resists the effects of the washing.
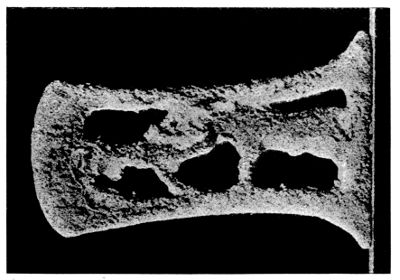
Fig. 41. Bronze axe-blade before treatment by Finkener’s method (Aeg. 13203).

Fig. 42. The same side after treatment.
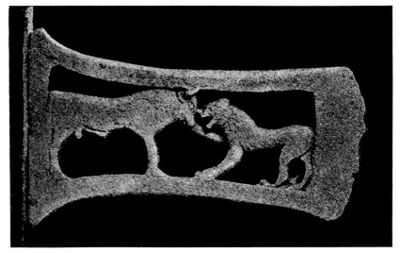
Fig. 43. Reverse side of axe-blade after treatment.
It is occasionally found that a bronze cannot stand the process of reduction, either because there is only a thin layer of metal over a stout core, or because the metal is permeated with cuprous oxide, which when tested with a file has a metallic appearance. The bronze must therefore be continually watched whilst it is in the cyanide bath, and if [135] necessary should be taken out even before the reduction is complete. This should be done if large pieces or large quantities of a powdery precipitate fall from the bronze, or if it is found that a needle readily pierces the oxidized layer. A specimen of this kind must be taken from the bath, carefully steeped, dried, and impregnated[148].
It is not to be expected that bronzes which are in an advanced state of decomposition (e.g. Figs. 9-12) can be so transformed by reduction as to appear as they did when they left the artist’s hand. For, although the decomposed oxidized layer is now reduced to metal, this no longer forms a coherent mass, but a loose powder, which, being deprived of its essential constituents, chlorine, oxygen and carbonic acid, no longer retains its coherency, but falls to the bottom. [136] Only in the interior and in the pores is the reduced metal retained.
In addition to the preservation of articles by the removal of the injurious chlorine compounds (as is also the case with Blell’s and with Krefting’s method for iron antiquities), the process may result in the discovery of inlaid work, inscriptions or ornamentation, the presence of which was not suspected. The accompanying illustrations (Figs. 39 and 40) show bronzes before and after the preservation process, while the axe-blade shown in Figs. 41-43 illustrates equally clearly the advantages which accrue from the treatment. Not less striking is the result of the treatment in the case of the dagger-sheath shown in Figs. 44 and 45 by which the design was discovered.
Reference may here be made to a case described elsewhere[149], in which reduction proved that what had been thought a single bronze object consisted in reality of two pieces which [138] did not belong to each other, but were fitted together by means of a bottle cork of modern date! In another instance a bronze was found upon reduction to be brazed with a hard solder containing zinc, which was thus quite inconsistent with the age ascribed to the object.
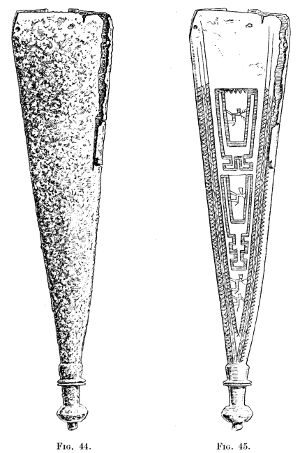
Fig. 44.
and Fig. 45.
Dagger sheath before and after treatment by Finkener’s method.
A short digression may be here made in order to discuss the question whether the composition of the bronzes undergoes any alteration. Three analyses[150] of Egyptian bronzes before and after reduction by Finkener’s method show that the change in composition is so slight as to be immaterial. It is of course obvious that greater differences will be seen in the results of the analyses before and after reduction of bronzes which are in an advanced state of oxidation, for in this case chlorine, oxygen, water, and carbonic acid constitute an appreciable proportion of the total weight. But even in these cases the analysis made after the reduction shows very slight variation from that of the original metal.
| Osiris | Osiris | Ibis | |||||
| Before | After | Before | After | Before | After | ||
| Reduction | |||||||
| Tin | 2·16 | 2·27 | 4·30 | 4·21 | 8·66 | 8·46 | |
| Copper | 77·83 | 77·45 | 79·66 | 79·74 | 88·53 | 88·75 | |
| Lead | 19·23 | 19·86 | 15·51 | 15·58 | 1·69 | 1·95 | |
| Iron | 0·12 | 0·14 | 0·28 | 0·24 | 0·21 | 0·20 | |
| Nickel & Cobalt |
0·29 | 0·24 | 0·20 | 0·17 | 0·30 | 0·29 | |
| Arsenic | 0·17 | 0·23 | 0·17 | present | 0·32 | present | |
| Antimony | trace | trace | trace | — | 0·20 | present | |
The two latter bronzes were tested qualitatively only for arsenic and antimony, and when the three objects were [139] washed the hydrated tin-oxide described on p. 130 was only found in the case of the Ibis. In this connection it should not be forgotten that slight differences in the quantities may be due to errors in the analysis as well as to a want of homogeneity in the alloy.
Krefting’s Method. This method is similar to that used for the reduction of iron (see page 108). The layer of oxidized material is removed in several places by filing, hammering, or rubbing with emery cloth until the metal is exposed. The object is then wrapped round with strips of zinc, and placed in a 5% solution of caustic soda. The hydrochloric acid produced in the process of reduction acts upon the soda to form sodium chloride. Here too the greatest care must be taken that the steeping is sufficient.
Personally I prefer Finkener’s method, for potassium cyanide is more easily washed out than soda, and also, although poisonous, is less caustic.
Krefting’s method however has proved of considerable success in some cases, notably in the treatment of some 40-50,000 Roman copper coins at the Berlin Museum. These were, with few exceptions, covered with a crystalline layer resembling green malachite or blue azurite and were quite illegible. Various unsatisfactory attempts were made to clean them with ammonia, with warm and cold acids of different kinds, with acid and iron nails, and by electric current both in an acid solution and in a solution of potassium cyanide. The following method finally proved satisfactory[151]:
[140] Krefting’s Method Applied to Oxidized Copper Coins.
“A thin plate of zinc with a bright metallic surface is perforated with a brad-awl, having a diameter of from 2 to 5 mm., until there are about 50 or 60 holes in each square metre. This is placed with the sharp edges of the holes uppermost on a row of glass rings (or crystallizing dishes will serve the purpose) 20 mm. in height resting upon the bottom of a large glass vessel. The coins, which in this case were 20 mm. in diameter, were then placed on the zinc plate, so that 7 or 8 of them occupy a space of 1 square decimetre. Another similarly perforated plate is laid upon them, and upon this more coins are arranged in the same way, and so on until there are six or eight double layers. A perforated zinc plate is then placed on the top with the sharp edges of the holes turned downwards, and over this a few zinc plates which have been previously used. The whole pile is surmounted with weights or stones resting upon glass rings or inverted glass dishes in order to press the sharp edges of the holes into the closest possible contact with the coins. A 5% solution of caustic soda is then poured over the whole, the immediate result of which is an evolution of gas. The reduction of the coins is usually complete in fifteen to eighteen hours, after which they should be well washed. After several rinsings in cold water they are placed, about 1000 at a time, in a large vessel fitted with a perforated false bottom containing hot water, which should be renewed three or four times every day. After four days the coins are wiped with a cloth and thoroughly dried on a warm oven plate [141] or in a drying chamber at a temperature of about 212°F. [100°C.]. They are then brushed with a bristle brush before a dust extractor, a procedure rendered necessary by the fine metallic dust from the coins, which then assume a light or dark brown colour such as is seen on copper coins which are in actual circulation. The practice of placing the coins whilst still wet into melted paraffin wax at 260°F. [120°-130°C.], which gives a dark appearance even to the brightest, has the disadvantage that the wax prevents the use of sealing-wax for taking impressions, and is therefore not recommended.
The reaction is analogous to that which occurs in the reduction of iron. The copper of the coin forms in the alkaline solution an electric couple with the zinc, and the hydrogen which forms at the copper end reduces the copper compounds covering the coins to metallic copper, and thereby loosens them, while the zinc oxide which is simultaneously formed is dissolved in the soda solution. In actual practice a part only of the zinc oxide is dissolved, while the remainder forms a white coating on the zinc [152]. Experience shows that a 4-5% solution is the most suitable for this method of reduction, which gives the most favourable results when these details are followed. If for example the zinc plate is laid immediately on the bottom of the glass trough, if the coins are laid too close together on the plate, or if there are more than 6 to 8 double layers in a trough, the process [142] of reduction is often incomplete, and it is then necessary to treat the coins a second time. It is scarcely necessary to mention that larger coins must be placed at proportionately greater distances from each other.
The 40-50,000 coins which were thus treated had originally been tinned, but the tin only remained at a few places. When the coins were washed immediately after the reduction, this tin could still be clearly distinguished, but on further washing, drying, and brushing, it ceased to be visible on account of the dark colour imparted to it by the finely powdered copper. In one [143] or two cases lead appeared on the surface of the coin, but was easily removed by mechanical means.”
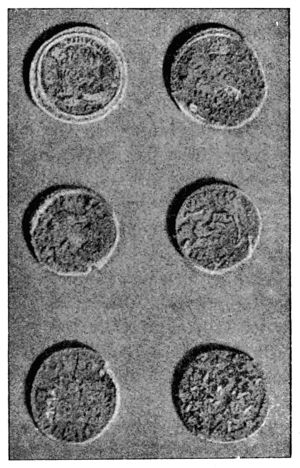
Fig. 46. Roman coins before treatment.

Fig. 47. Roman coins after treatment by Krefting’s method.
Cleaning Copper Coins by Melted Lead.
Although the results obtained by this method are less satisfactory than those produced by the preceding, it has the advantage of simplicity[153].
[144] “Using a pair of tongs, dip the coins one by one into melted lead until the crackling, which begins at once, has ceased, which occurs in from 3 to 10 seconds. The hand should be protected with a glove from the spluttering molten lead. The coin is then thrown into cold water, cleaned, and placed until the next day in hot milk. It may be necessary to repeat the process when the coin has become cold. By this method an olive colour is imparted to the coin which many antiquaries prefer to dark brown, but personally I prefer Krefting’s method because it renders the inscription and designs far more distinct. A coin which after the treatment with melted lead has remained so covered with cupric oxide as to be still illegible can seldom be improved by a repetition of the treatment, whereas had the zinc treatment been applied in the first instance the result would probably have been satisfactory. This conclusion seems to be justified by the extremely small percentage of coins which, in my experience, have remained illegible after the treatment by electrical methods.”
(C.) Preservation of Bronzes by the Exclusion of Air.
In those cases in which the advanced state of decomposition renders the reduction process either inapplicable or at any rate inadvisable, or in which the decay is not likely to be arrested by impregnation, a further method of preservation remains, viz. the complete exclusion of air and moisture.
If air is completely freed from moisture the oxygen can no longer act in conjunction with the copper chloride upon the still intact metal (see page 29 et seq.), and the condition of the bronze will consequently remain unchanged.
[145] A bronze, for example, which shows much decay should be placed after impregnation under a hermetically sealed bell glass, and beneath or near it should be placed some dehydrating agent, of which anhydrous calcium chloride is the most suitable (see note, p. 123). To exclude the air completely the bell glass should have a projecting ground edge, which should be smeared with vaseline or grease and pressed firmly upon a thick well polished glass plate. The dehydrating agent may be placed in a glass vessel or dish in such a way as to be unseen, or it may be covered with two or three [146] thicknesses of dark gauze or with black cardboard laid loosely over it. If an object is too large for a bell glass, or if several objects are to be exhibited together, a square plate-glass case with iron framework, made air-tight with putty, may be used as shown in the illustration (Fig. 48). The lower part, containing calcium chloride, is partitioned off by a perforated plate covered with black gauze[154]. A hygrometer was placed behind the head, the indicator of which has remained at zero since it was first fixed several years ago, and the bronze has not hitherto shown any sign of change, although the inlaid gold is in parts raised from the metal by a light-green oxychloride. The cost of these cases is considerable, but for valuable objects this should not be considered. In the place of calcium chloride, sticks or lumps of caustic soda may be used with advantage, for this substance absorbs both moisture and carbonic acid.
Fig. 48. Method of mounting objects in air-tight cases.
This method of preservation is of course applicable not only to decomposed bronzes but to all valuable antiquities, whatever the material may be.
These methods are founded upon the fact that the sunken areas of the coin are, by the pressure of the die in stamping, rendered denser than the raised portions, such as the inscription. The earliest method is that published by Brewster, reported by Süpke[155]. The coins when cleaned are placed upon red-hot iron, which causes the oxidation of the entire surface [147] of the coin. The thin film of oxides varies in colour according to the duration and the intensity of the heat. The oxidation of the letters of the inscription differs from that of the surrounding parts, and is recognisable by a difference in colour. Drude [156], treating more especially of silver coins, remarks that the inscription is rendered legible by heating them to redness over a Bunsen-burner. It then, according to “Prometheus[157],” when viewed in a dark room, appears dark on a bright ground, especially if the coin has been previously polished and then roughened again by slightly etching it with acid. In conclusion, the method of Roux[158] may be quoted:
“The smooth-worn and polished coin is placed in a solution of copper sulphate or of some other metallic salt, and suspended between the electrodes of one or more cells of a battery (any other form of continuous current will serve the purpose). If the current is weak, the electrodes must be near to the coin. The stronger the current the more rapidly the impression appears. On the side which faces the anode or positive plate the impression is metallic; on the other side, after gently wiping off the less firmly adherent part of the oxide, the impression appears in grey lines. These markings can be fixed by varnishing them with a thin alcoholic solution of shellac. To render the impression legible on both sides, the coin should be placed upon the four upturned feet of an insulating stand. The larger the coin the deeper must be the layer of solution above and below the coin. The depth below should be equal to the radius of the coin.
This can perhaps be most conveniently carried out by placing that electrode in immediate contact with the [148] coin which upon immersion in the solution becomes tarnished with the metal, i.e. the cathode or negative pole. Other portions which it is not intended to treat should be first covered with varnish.
The striking success of this method is due to the fact that that portion of the metal which has been compressed by the stamp is a better electrical conductor than the rest; no success could therefore be expected from the use of this process for the restoration of such objects as worn engraved copper-plates, etc.”
Preservative treatment of silver is scarcely necessary (cp. pp. 49-52), except in those cases in which the silver is alloyed with a large percentage of copper, and which show efflorescences similar to those which appear upon bronzes containing chlorine. Electrolytic reduction will be found to be the most suitable method of treatment in such cases. To treat silver coins they should be placed in contact with iron nails in lemon juice. Instead of the citric acid, which is the active principle in this process, other diluted acids and other metals, e.g. zinc, may be employed. Flinders Petrie[159] has shown that the reduction can also be effected by a weak solution of common salt. Silver chloride is soluble in ammonia, and thin layers may be removed by the application of ammonia by means of a soft brush. Thorough rinsing with pure water, drying with soft cloths, and cautious warming are always essential.
An excellent reducing agent for single coins, the characters of which are rendered illegible by a layer of silver chloride, is molten potassium cyanide, or a mixture of this substance with sodium or potassium carbonate. In a short time the silver chloride is decomposed and removed from the smooth [149] surface of the coin. After boiling out with water, steeping in alcohol, drying, and brushing with a soft brush, the coins may be coated with zapon. Coins treated in this way appear to be less brittle than those reduced by Krefting’s method. More troublesome but less dangerous, because potassium cyanide is not used, is the treatment of silver coins with a fused mixture of potassium and sodium carbonates. In this case the silver chloride is converted into silver carbonate, which is then decomposed with 50% acetic acid. Further treatment by washing, drying, and impregnation is carried out as previously described.
Silver which has become friable (p. 51) can be rendered more compact by cautiously heating it to redness. It will however be advisable to entrust heating and mechanical treatment of objects which are much bent to some skilled silversmith, whose experience may prevent disaster. Silver objects which are largely converted into friable chloride, especially if they are much expanded, or if large portions have broken away in the process of removing the chloride, will hardly bear any other treatment than that of impregnation with gum-dammar solution or with paraffin wax. As silver chloride is easily fused such articles should not be subjected to heat.
Earthy matter can often be removed with a neutral soap and warm water, while calcareous accretions can be dissolved by a 2% solution of hydrochloric acid. Silver which has been blackened by silver sulphide may be laid in a warm 2% solution of potassium cyanide. All objects should be subsequently well washed with warm water.
Objects of pure lead and pure tin are rare. If much oxidized they should be washed with warm water, dried, and impregnated with a gum-dammar solution or with paraffin [150] wax (pp. 70 and 91). If in a good state of preservation they may be freed from any earthy or calcareous coating or from lead carbonate by the cautious use of very dilute nitric acid followed by steeping in water.
Ceresole [160] cleans oxidized leaden seals with 10% acetic acid, neutralises the acid with ammonia, and after five minutes in alcohol coats them thinly with wax. The seals are preserved between glass dishes (Petri dishes), the space between the dishes being filled with cement. I employ Krefting’s method for leaden medals, using either zinc and very dilute sulphuric acid, or zinc dust and caustic soda. Occasionally the zinc dust becomes firmly cemented by oxide to the surface of the lead, and, if this is the case, great care must be used in removing it. The washing process also requires care. A very efficacious method is to allow a stream of warm distilled water, from which the dissolved air has been driven off by boiling, to flow over the object in a porcelain dish. I now omit any impregnation with paraffin wax, and instead recommend removal of the water by alcohol, drying, and coating with zapon. To preserve the specimens after treatment, more especially from the injurious action of perspiration from the hands, they are placed between dishes of glass or of celluloid[161].
Objects of pure gold usually need only be cleaned with soap and water and a soft brush; lime may be removed by the application of a 2% solution of hydrochloric acid. A coating of silver chloride occurring on gold which contains a large percentage of silver may be removed by ammonia, or, [151] in certain cases, by the alternate use of dilute hydrochloric acid and ammonia.
A layer of red ferric oxide (see p. 53) is of frequent occurrence upon gold objects, and may be removed by warming the object in a stronger solution of hydrochloric acid, but soft brushes will often serve the same purpose. Pure gold being very soft, only the softest so-called “silver brushes” should be used, and all pressure or bending should be avoided. If friable the object should be carefully impregnated with a solution of gum-dammar (p. 70).
If covered with a film of dirt, or if when in a collection objects of glass or enamel undergo any alteration, they should be washed or steeped in lukewarm water. When dry they should be treated with pure olive oil or poppy-seed oil, which may be diluted with benzine. The oil helps to restore the lustre to the glass and to bring out the colour of the enamel. When thus treated the objects should be carefully protected from dust.
A decomposition of ancient glass when deposited in a museum has been hitherto only rarely observed, but allusion may be here made to the so-called ‘sweating’ of glass which is a question of considerable importance in Industrial-Art collections. In this case preservation is insured by washing with distilled water, drying, and coating with zapon. Further particulars may be obtained from the paper by Pazaurek[162].
Many curators dry carefully and impregnate them with a gum-dammar solution or shellac; isinglass or glue are however preferable, for these aqueous solutions may be used for [152] the treatment of damp objects, which could scarcely be dried without cracking. In order to permeate the object these solutions must be very dilute, and are most advantageously applied at a temperature of about 120°F. [50°C.]. The impregnation may also be effected in rarefied air under a bell glass (p. 68). Friable bones and similar objects which might fall to pieces in the solution during impregnation should be bound with strips of gauze or with string before immersion; they are easily removed when cold. To prevent the formation of mould a small quantity of dissolved corrosive sublimate[163] is added to the glue, or when dry after impregnation the objects may be covered with a solution of shellac or resin. Impregnation is of very general application, and is frequently used for the preservation of fossil and pleistocene bones.
At Copenhagen the method used to render leather soft and pliable is to place it in train oil for an hour and then dry it with filter-paper. Lanoline may also be used with success [164]. Poppy-seed oil in benzine (p. 86) is said to produce [153] good results, but the “Merkbuch” recommends the preservation of leather in this condition in alcohol[165].
Earth and soil may be removed by mechanical means, and, occasionally, careful washing may be possible. The objects should be dried and impregnated with a gum-dammar solution (p. 70), poppy-seed oil (p. 86), or a solution of india-rubber (p. 90), or they may be preserved in alcohol (p. 159). Some textile fabrics in the Copenhagen Museum owe their excellent state of preservation to Steffensen’s treatment, i.e. impregnation with a solution of india-rubber in turpentine with the addition of bees’-wax.
The following account of the treatment of textile fabrics from the Lake-Dwellings is due to Herr Heierli, of Zürich:
“The pieces as they were taken up were laid on the ground and thus slowly allowed to dry in the air. They were then placed between glass plates, the edges of which were pasted over with paper. Old pieces which had been dry for a long time, and which had become tender and friable, were laid on the ground and watered from time to time until they were soaked through; they were then treated in the manner already described.”
[154] Egyptian textile fabrics preserved between glass plates often deposit a thin layer of salt on the glass, but this is easily wiped off (see p. 155). It must first be ascertained by a previous trial in each case whether the salt can be removed by steeping in water or in alcohol and water.
Hair found in peat has always a dark-brown colour from impregnation with peaty matter. The method proposed by Bille Gram [166] for restoring the natural colour consists of repeated and alternate treatment with very dilute alkali solution and acid at about 120°F. [50°C.]. When the liquid ceases to show coloration the natural colour of the hair is restored.
These do not require any treatment beyond protection against insects, which is attained by immersion in an alcoholic solution of corrosive sublimate, or by spraying with corrosive sublimate in either alcoholic or aqueous solution. Of course the poisonous nature of corrosive sublimate necessitates caution in its use and it should be always labelled as such.
The use of naphthalene is not always successful, and white scales of naphthalene are apt to make their appearance; nor does finely powdered pepper sprinkled on the feathers, either alone or mixed with finely powdered alum, give satisfactory results.
The method of cleaning and preserving papyrus in use in the Egyptian department of the Royal Museums at Berlin is as follows: Those pieces which are folded together or rolled are carefully straightened, and, if very friable, they are first placed between damp filter paper to render them uniformly [155] pliable. Dust and dirt are removed with soft paint-brushes, crystals of salt which are often found[167] are picked off with forceps. Any growths of fungus are carefully scraped off with a knife. The papyrus thus prepared is then placed between two thick polished glass plates, the two opposing surfaces of which are covered with a very thin layer of vaseline. Air is frequently admitted to dry the papyrus, while the pressure of the glass plates tends to smooth it out, and after it has been so treated it is mounted between thin glass plates, the edges of which are pasted over with paper covered with an oil paint.
A papyrus preserved between glass plates often shows round the edges a whitish border about two millimetres in breadth, and on separation the glass plates show a slight film of the same white material on the surface which had been in contact with the papyrus. The formation of this film, which consists chiefly of common salt and is easily wiped off, may be prevented by previously washing the papyrus in distilled water, a proceeding which experience has shown to be harmless. As the papyrus will swim on the surface it should first be immersed in alcohol until soaked through; the process of steeping is then quite simple. The thinness of papyrus enables the steeping to be completed after 24 to 48 hours by two changes of the water, and care must be taken lest a too prolonged steeping should obliterate the lettering. The water assumes a yellowish or brown tint and the [156] papyrus becomes somewhat lighter in colour on drying. Papyrus may also be preserved by zapon (see Appendix), but this method has no advantage over that of mounting between glass plates.
To preserve adequately articles of moist wood (and they are generally in this condition when first excavated), preliminary measures to prevent their drying in the air must be taken immediately after they are dug out of the earth. If found in water, as for instance articles from pile-dwellings, they should be conveyed in water; moist objects should be wrapped in several thicknesses of moist cloth, and the whole wrapped in gutta-percha membrane, or in a layer of moist moss. The cracks which arise in wooden objects which have become dried may frequently be closed up by laying them in lukewarm water.
As the earliest attempts at preservation were probably made upon wooden objects there is scarcely a collection in which a number of methods are not employed. One exception only is known to me, and here, after a plaster of Paris cast has been taken, the object is simply allowed to shrink. The methods proposed and carried out are so different and so numerous, especially as regards the liquid used for impregnation, and in such variety, that it is only necessary to deal with the most important. These may be divided into two classes, viz. dry and wet.
(1) Dry Preservation of Wood.
Moist or wet objects are placed in thin size or in a solution of isinglass till they are impregnated, after which they are dried gradually in a shady place. A solution of shellac, or varnish diluted with petroleum or benzine, is then put on with a brush.
[157] Sometimes the objects are placed directly into a mixture of varnish and petroleum, or they are impregnated with melted paraffin. The former is preferable as a means of impregnation if there are cracks or holes, for the superfluous solution readily drips from the wood when it is taken out, while paraffin sets too soon to drain out of the cracks, and thus imparts an unnatural white appearance to the wood. Owing to the large size of the vessels which would be otherwise required, paraffin is only useful for small or medium-sized objects, but when making use of varnish one end of a large object [168] may be placed in the mixture while the solution is repeatedly poured over the object. After two or three days the opposite end should be placed in the solution. By repeating this process every part of the object will soon be thoroughly impregnated.
Objects of still greater size, such as a Viking’s ship, can only be preserved by painting the surface. In such cases it is advisable to begin with dilute varnish so as to allow the impregnating solution to penetrate as deeply as possible into the material, instead of merely forming a skin.
A solution of waterglass has in one instance been used for the preservation of a large boat, but the result is not satisfactory.
Leiner’s Method[169]. The wooden articles are laid in glycerine mixed with a small percentage of carbolic acid. The length of time during which they remain in the glycerine depends upon their size. When taken out they are lightly wiped and preserved without further treatment. If a growth of mould should occur it may be washed off.
Objects thus treated retain their moist condition and should therefore be very carefully protected from dust.
[158] Speerschneider’s Method[170] (cp. p. 91). Small specimens are heated for two hours in a mixture of
8 parts of rape-seed oil,
1 part of bees’-wax,
1 part of pine resin, and
2 parts of benzene.
Larger objects require a proportionately longer heating, but the mixture must not be allowed to actually boil. The moisture rises as steam and causes the solution to bubble. The bubbling however continues after the moisture has been driven off; great care must therefore be taken that the heating is not so prolonged as to cause the object to shrink. The highly inflammable nature of the mixture renders great caution necessary, and should it ignite, a lid, which should always be in readiness, should be put on the vessel. After impregnation the objects are wrapped in blotting-paper and laid in ashes for four days to prevent the access of air. The aim is doubtless to insure thorough absorption of the superfluous liquid which remains upon the object, which exposure to air would prevent by causing the mixture to set too rapidly. The same mixture can be used repeatedly, but each time two-thirds of the original quantity of benzene must be added.
Herbst’s Method[171]. The moist objects are boiled in a saturated solution of alum for two hours (hot water dissolves about 31⁄2 times its weight of alum), but if they are of some thickness the time must be proportionately longer. They are then taken out, and when the alum in crystallizing has made them more or less firm, the crystals adhering to the surface are washed off with warm water.
When thoroughly dry the wood is brushed over with hot [159] linseed oil, which operation is repeated until no more oil is absorbed. A final thin coating of varnish or shellac is then given. According to Steffensen, the method followed at Copenhagen is to lay the objects in warm thin size for a quarter of an hour after impregnation with alum. This alum-method is there used for objects of oak, although the “Merkbuch” (p. 60) states that only the varnish-petroleum mixture should be used for impregnating this class of object.
(2) Preservation of Wooden Objects in Liquids.
The expense entailed by this method renders it applicable only to articles of small size.
The preservation of small objects in a flat vessel, the bottom of which is covered with glycerine, has the disadvantage that glycerine extracts organic substances and thus assumes a brown colour. If glycerine is used the object should undergo a thorough preliminary steeping, and the glycerine should be renewed until it remains colourless. Closed cylinders filled with glycerine or a mixture of glycerine and water are not convenient because wood nearly always floats in the liquid. This may be remedied however by the addition of alcohol.
Jenner’s Method[172]. When the objects have been thoroughly cleaned with water, pure alcohol, diluted with water until the specific gravity at 54°F. [12·5°C.] reaches 0·96, is poured over them. After six or eight weeks the alcohol is poured off and replaced by fresh alcohol of the same specific gravity. This alcohol is examined in a year’s time, and should always show a specific gravity of 0·96. The [160] alcohol which has been poured off may be filtered, and if necessary decolourized by animal charcoal; when the specific gravity has been again raised to 0·96, by the addition of fresh alcohol, it may be used again.
The same process is applicable to textile fabrics, yarn, and leather.
Protection against Wood-worms, etc.
All the methods mentioned above will destroy insects and their larvae.
In cases in which it is either impossible or undesirable to use immersion or external application, as for instance in the treatment of objects of dry wood, the larvae may be destroyed by dropping petroleum, an aqueous solution of potassium arsenite, or corrosive sublimate, into the various small openings. This will also help to prevent further attacks.
If solutions are not applied insects may be destroyed by the vapour of carbon bi-sulphide or of crude benzene. These liquids, which are sufficiently volatile at the ordinary temperature, should be placed, together with the objects to be treated, in a closed box.
I have used a similar method for the destruction of wood-worms in Egyptian coffins. The coffin is placed in a large wooden box lined with tin plate. The lid, also lined with tin, is provided with projecting edges, to which strips of felt are glued. The weight of the lid by compressing the felt is sufficient to render the box air-tight. Six or eight glass vessels containing crude benzene are placed at the bottom of the chest and of the coffin itself. It need scarcely be added that the box must not be opened near a fire or light, as the vapour forms an explosive mixture with air; it is in fact advisable to have no light or fire in the room.
Insects can also be killed by naphthalene vapour, but as [161] naphthalene is insufficiently volatile at ordinary temperatures, the method above described is more convenient[173].
Preservation and Cleaning of Coloured Wooden Objects.
For objects of this kind materials should not be used which, like varnish, tend to darken and so to damage the colours. Gum-dammar solution (page 70) answers the purpose, but colourless collodion is better. Colours which are soluble in water (as is frequently the case with wooden objects from Egypt) cannot of course be cleaned with water, but benzine may be applied by means of soft cloths or brushes. Resinous or pitch-like substances may often be removed from coloured objects by turpentine mixed with benzine or ether.
A method of cleaning gilded or brightly coloured ecclesiastical figures which is used in the Breslau Museum is the application of a mixture of copaiba balsam and ammonia. This method is similar to that used to clean paintings[174], the action of the solution being that of a mild soap.
Antiquities which were originally uncoloured, but which have been subsequently painted, may be cleared of paint by means of a solution of caustic soda in water or alcohol. [162]
After the mechanical removal of any adherent earth and dust, the specimen should be rubbed carefully backwards and forwards between the fingers covered with a soft woollen glove. Particles of soil should be picked out of any holes and indentations by using a strong horse hair[175]. It is then preserved by impregnation with a solution of shellac, poppy-seed oil, or isinglass (pp. 70 and 86).
The following particulars of the method used in Messrs Stantien and Becker’s collection of amber have been supplied by Prof. Klebs:
“Amber is preserved best in distilled water: I add a very small quantity of glycerine and a still smaller amount of alcohol. A proportion of alcohol greater than 1% is injurious to the amber. A thick layer of gelatine containing glycerine is an excellent medium for the preservation of large objects if they are kept free from dust. This layer should be washed off and renewed every few years.”
In addition to the protection from dust afforded by closed glass cases, it is also important to protect objects from the action of direct sunlight, especially during the summer months. There is, for instance, no doubt that the decay of bronzes, even of those with a patina which is apparently sound, is hastened by the great variations of temperature, caused by the rays of the sun falling directly upon them. Similarly objects which have been preserved by the application of solutions of resin or varnish should be protected from the direct access of [163] sunlight, for the sudden warming may easily cause cracks. Nor should antiquities be kept near the heating apparatus. There is another precaution, to which too little attention has been paid, viz. the protection of objects as far as possible from even diffused daylight. Although no investigations upon the extent of the injurious action of light have as yet been published, light is not without influence upon the outward appearance, and therefore also upon the material condition of antiquities of organic origin. But even inorganic objects, such as pigments, glass, enamel, amber, etc., are affected by light; it is therefore certainly advisable to protect antiquities of all kinds from light during the time in which they are not exhibited to the public.
The public is effectually prevented from fingering antiquities which are enclosed in glass cases, but it may be well to remind those who have to handle them in the course of their duties that contact with the bare hand can only be harmful, even though fingering is understood to be beneficial to modern bronzes by inducing the formation of patina. The bright surface of metallic iron which results from treatment by Blell’s or by Krefting’s method, especially if there is a thin coating of paraffin, should not be touched at all with the bare hand, but only with a cloth or a glove. Bronzes, whether intact or restored, and iron objects, should never be in direct contact with those which show efflorescences.
The usual custom is to attach labels of painted cardboard or metal by means of thin metal wire. The tendency to rust makes iron wire unsuitable, especially for objects containing salt, which are quickly affected; thus light coloured earthenware may soon be covered with spots of rust. Copper wire and nickel wire are liable to be similarly attacked. Many years ago it was noticed[176] in the Ethnological Museum at Berlin that nickel wire when in contact with silver objects [164] which were covered with silver chloride was destroyed by the formation of a deliquescent green nickel salt. Silver or platinum wire forms the most suitable means of attachment, but if the expense of these is too great, copper or nickel wire may be used, except in the cases mentioned above.
Small objects of any kind, which one still frequently finds kept in open cases, are better preserved in upright glass cylinders with glass stoppers, or in cheaper glass tubes, one end of which is fused and the other closed with a cork.
The methods of preservation which have been described in the preceding pages may be thus tabulated and summarised:
| Methods. | Application. |
| Steeping in water, drying and impregnation. | Limestone, Earthenware, Iron, much corroded. |
| Direct impregnation. |
(1) Unbaked earthenware, etc., (2) Bronze objects with little or no metallic core, or showing a cracked or warty surface, (3) Objects of wood and of other organic substances. |
| Removal of compounds of oxygen or chlorine | |
| (a) by chemical process, | Iron objects in a good metallic condition, |
| (b) by electrolytic process. |
(1) Iron objects with a sound metallic core, (2) Bronze objects with a sound metallic core. |
| Mounted thoroughly dry and hermetically sealed. | Valuable bronzes in an advanced state of decomposition. |
There will be no difficulty in the choice of methods for [165] limestone or earthenware, whether kiln-dried or sun-dried, for a simple experiment will prove whether steeping is likely to cause injury or disintegration.
The methods are themselves simple and inexpensive. For organic substances the chief question is the choice of the most suitable medium for impregnation.
Iron and bronze present some difficulty, although the use of a file will readily show whether reduction is feasible.
The simplicity of the apparatus required for Krefting’s method gives it an advantage over other methods, at any rate for iron objects. Objection has been taken to the methods of reduction, because they give to the objects thus treated an appearance to which the public are not accustomed. It may be safely asserted however that this appearance more truly represents the object when in actual use, than the oxidized and rust-covered specimens to which we are accustomed in antiquarian collections. To those who value an antique object for the crust that covers it, all methods of restoration must be objectionable. Such persons ought to object to the removal of the incrustations which hide the cuneiform inscriptions on clay tablets. On the other hand, those who regard these methods with approval should go a step further and confide their collections to experienced hands for some form of treatment which may bring to light inscriptions and inlaid work which will greatly enhance their value.
To spread the knowledge of these methods and to invite the co-operation of others is the aim of this book. As to the best method to be used in each particular case it is unnecessary to lay down any hard and fast rule, for this can only be learned by observation and experience. [166]
For this purpose a proper brush is required with strong bristles, closely set as in a scrubbing brush; the brush should have a firmly fixed handle, preferably slightly curving upwards to save the knuckles from being bruised upon the stone. A so-called “silver brush” will serve the purpose. The paper should be stout and stiff enough to resist the blows of the brush without tearing. An admirable paper, which possesses these qualities, is specially prepared for the purpose by the O.W. Company, 100, Great Russell Street, London, W. As a substitute for the specially prepared paper stout packing paper may be used with satisfactory results.
The stone should be tilted if possible at an angle of about 45°, and the surface bearing the inscription should be well washed or carefully scraped free of dirt and foreign matter and should be rendered thoroughly wet. A piece of the special paper of suitable size should be soaked in water for a minute or more. It should then be carefully applied to the surface of the stone in such a way as to prevent air-bubbles. This may be assisted by gently smoothing it with the hand or back of the brush. When close adhesion has been secured, and all air-bubbles removed (this can sometimes be done by pricking through the paper with a pin), the paper should be sharply beaten with the brush, the blows being delivered from the wrist and not from the shoulder [167] until it begins to show a fluffy appearance. It should then be peeled off and allowed to dry, after which it may be rolled or folded without danger of injury to the embossed inscription.
Should the paper tear, another piece soaked as before may be placed on the top and beaten until it becomes incorporated with the first. If the letters are large and deep, or if the surface is much cracked, two or more sheets superimposed should be used. In the case of large inscriptions it is advisable to take impressions by sections, care being taken that each sheet slightly overlaps the preceding one to prevent the possible omission of some of the letters.
It is also useful to take at the same time a pen or pencil copy of the inscription, for a comparison of the copy and the squeeze will often prevent errors in deciphering. The squeezes can be very well deciphered by artificial light, while doubtful letters may sometimes become clear on holding up the sheet to the light. The reverse side of the squeeze, upon which the inscription stands in relief, may afford great assistance when read by the aid of a mirror. A photograph of the squeeze will often reveal more than a photograph of the inscription itself.
The method is described by S. Reinach in his “Traité d’Épigraphie Grecque” (Introduction, p. XX. ), where he also refers to Hübner, “Ueber mechanische Copien von Inschriften,” 1871. [168]
Further particulars may be given of the new preparation known as Zapon. This substance is now made on a large scale, and can be obtained from the British Xylonite Co., Brantham Works, Manningtree (Xylonite lacquer F. 6631). The following excerpt is from a short communication in “Prometheus” (XV. 1904, pp. 485 and 499), which deals with the preservation of wax seals and of glass.
Zapon, the invention of Crane, of Shorthills, U.S.A., has been used for 20 years past for the protection of metals from oxidation and the action of sulphuretted hydrogen. Although the products of the various manufacturing firms differ in composition, zapon is essentially a solution of nitro-cellulose in various solvents. The nitrated cellulose, i.e. gun-cotton (pyroxyline), is generally, with the addition of camphor, dissolved in a mixture of amyl acetate (hence the peardrop-like smell) to which distillation products of petroleum, etc., are added. It comes into the market as a faintly yellow, slightly oily liquid. Its use as a preservative depends upon the fact that the evaporation of the solvent leaves behind it a fine transparent coating of gun-cotton (pyroxyline). Zapon for preservative purposes must have a neutral reaction, and must not under any circumstances redden litmus paper. Its use in this connection is due to Schill, who also recognised its suitability for other materials, as, for example, for plaster casts, the treatment of [169] which is eminently simple, for it consists in dipping small casts, or in painting larger ones with a soft brush. It is advisable to begin at the top and apply it from above downwards, using a clean dry cloth to wipe off any excess of the fluid which collects in the deeper parts of the cast. If zapon containing about 4% of gun-cotton is used, the coating left on drying is scarcely visible; with a 5% solution a certain degree of polish results. Casts treated with zapon are less easily damaged by dust than those untreated, and may be cleaned with soap and water without injury to their surface, provided that a soft brush is used, but brushes which are stiff enough to injure the zapon coating will damage the contours of the statue. It should only be used for objects kept under cover, for rain and wide variations of temperature will attack them almost as readily as untreated casts. It can be used with equal success for antiquities of stone, clay, baked or unbaked, or for plaster after the soluble salts have been thoroughly removed by steeping, for if this has not been done the salts will soon crystallize out and loosen the protective coating. For objects which are free from salts impregnation with zapon possesses the advantage that it renders them less liable to damage from handling or dust, whilst the appearance is scarcely altered, if at all. This applies also to antiquities of metal, for unless the injurious chlorine compounds are removed by simple steeping, or reduction and subsequent steeping, treatment with zapon is useless. To bronzes, which in spite of mechanical cleaning show a somewhat unpleasant grey non-metallic appearance, zapon often imparts a distinct metallic lustre. To enhance this lustre by a second vigorous application is not recommended, for this gives the impression of a varnish. To protect articles of silver from the blackening influence of sulphuretted hydrogen, zapon is very useful, but does not afford absolute protection unless it has been thickly applied. In collections of armour [170] much use may be found for this material. The objects are dipped and then placed in a drying oven at 105°F. [40°C.] to secure rapid drying and uniform distribution. The amyl acetate or other solvent is best conducted away, as it evaporates, into a flue or into the open, although the vapours can hardly be considered dangerous to health.
The following references will afford some information on the use of zapon in the preservation of Archives:
E. Schill, “Anleitung zur Erhaltung und Ausbesserung von Handschriften durch Zapon-Imprägnierung,” Dresden, 1899.
O. Posse, “Handschriften Konservirung,” Dresden, 1899.
G. Sello, Das Zapon in der Archivpraxis (“Korrespondenzblatt des Gesamtvereins der deutschen Geschichts- und Alterthumsvereine,” L., 1902, p. 195).
Schoengen, Over hat Zapon (“Nederl. Archivenblad,” 1902, 1903, Nos. 1 and 3).
J. Perl, Das Archiv-Zapon (“Korrespondenzblatt,” LII. , 1904, pp. 119 and 435).
G. Sello, Die bei der Zaponverwendung in der Archivpraxis gemachten Erfahrungen (“Korrespondenzblatt,” LII. , 1904, p. 439). [171]
| A | B | C | D | E | F | G | H | I | J | K | L | M |
| N | O | P | Q | R | S | T | U | V | W | Y | Z |
CAMBRIDGE: PRINTED BY JOHN CLAY, M.A. AT THE UNIVERSITY PRESS.
FOOTNOTES:
[1] “Lexikon d. gesamten Technik,” Vol. I. p. 257. O. Lueger.
[2] “Merkbuch.” The excavation and preservation of Antiquities, 2nd edition, Berlin, 1894.
[3] “Mittheilungen aus der Sammlung der Papyrus Erzherzog Rainer.” Vol. I. p. 118. See also Flinders Petrie, Archaeological Journal, Vol. XLV. 1888, p. 88.
[4] Aeg. 105. [This and similar notes have reference to the catalogue of the Egyptian (Aeg.) or Antiquarian (Ant.) sections of the Berlin Royal Museums.] The limestone blocks were brought from the Mastaba of Meten, at Abusir near Memphis, explored by Lepsius in 1846. Meten was one of the chief officials under King Snefru, B.C. 2800. The inscriptions relate to his possessions and official career, while the pictorial representations depict hunting scenes and the offering of the gifts for the dead. The statue of Meten was found in the grave and is now in the Egyptian department (No. 1106) of the Royal Museum. Comp. “Ausführliches Verzeichniss der aegyptischen Alterthümer,” Berlin, 1899.
[5] Aeg. P. 4730
[6] Aeg. V. A. 2846.
[7] Aeg. P. 4739.
[8] It may be here mentioned that, as is well known to chemists, the efflorescences which often go by the name of “wall-saltpetre,” in most cases do not contain any saltpetre, but consist of sodium sulphate.
[9] Crum Brown, “Chem. Centralblatt,” 1890, I. p. 212; E. Simon, “Ueber Rostbildung u. Eisenanstriche,” p. 4.
[10] J. Spennrath, “Verhandlungen d. Vereins zur Beförd. d. Gewerbefleisses,” 1895, p. 245.
[11] “Christiania Videnskabs-Selskabs Forhandlinger” for 1892, No. 16, p. 8.
[12] “Chemische Zeitung. Repetitorium,” 1895, p. 289.
[13] “Chem. Centralblatt,” 1895, I. p. 441.
[14] Id., 1891, I. p. 860.
[15] “Berg- und Hüttenmännische Zeitung,” 1882, p. 469.
[16] [It may not be out of place here to give the main conclusions, drawn from a long series of experiments by Prof. W. R. Dunstan (“Proc. Chem. Soc.,” XIX. 150, 1903).
(a) Pure iron is not oxidised in the presence of gases and water-vapour only, but for the appearance of rust the presence of water in the liquid state is necessary.
(b) The reagents which prevent the rusting of iron are those in the presence of which hydrogen peroxide is decomposed, and which are consequently inimical to its formation: among such reagents the following are given—sodium chloride, sodium sulphate, ferrous sulphate and potassium nitrate.
(c) The action of H2O2 on metallic iron leads to the production of red basic ferric hydroxide, which is identical with ordinary rust. The composition of rust may therefore be represented by the formula Fe2O2(OH)2, the reaction being represented by the equations:
Fe + O2 + H2O = FeO +
H2O2.
2FeO + H2O2 =
Fe2O2(OH)2.
These views are however combated by Moody (“Proc. Chem. Soc.,” XIX. 157 and 239) who concludes that aerial rusting must be regarded as a change involving the interaction of iron and carbonic acid and the subsequent formation of rust by oxidation of the ferrous salt.
He also states that those salts which do not combine with and which are not decomposed by CO2 have no retarding influence on the formation of rust, e.g. sodium chloride, sodium sulphate, etc.
On the other hand substances which absorb and combine with carbonic oxide (e.g. sodium carbonate or hydroxide, ammonium carbonate, calcium hydroxide), or which are decomposed by carbonic acid (potassium and sodium nitrites), inhibit rusting, which may therefore be regarded as a change involving the interaction of iron and acid and the subsequent formation of rust by the oxidation of the ferrous salt.
O. Kröhnke (“Wochensch. Brauerei,” XVII. 233) gives the following equations:
Fe + 2CO2 + H2O = Fe(HCO3)2 +
H2.
2Fe(HCO3)2 + O + H2O =
2Fe(OH)2 + 4CO2.
Comp. also Dammer, “Handbuch der anorg. Chem.,” Vols. III. and IV. (supplement).
Considerable attention has also been directed to the influence of bacteria upon iron. Thus the growth of Crenothrix may cause much trouble in waterworks, vide “Centralblatt für Bakterien und Parasitenkunde,” II. 12, 681. A variety, Chlamydothrix (Gallionella) ferruginea (Mig.) appears to play an important part in the formation of rust (comp. Zopf, “Crenothrix polyspora die Ursache der Berliner Wasser-Calamität,” Berlin, 1879. De Vries, “Unter. der Crenothrix Commission,” Rotterdam, 1887 and 1890).
Neufeld (“Chem. Centralblatt,” 1904, I. 1621—abstracted from “Zeitschrift für Untersuchung der Nährungs- und Genussmittel,” VII. 478) gives particulars of three varieties: Crenothrix polyspora, which separates iron; Cr. ochracea, which separates aluminium and some iron; and Cr. manganifera, which separates manganese.
Jackson (“Journal of Society of Chemical Industry,” 1902, p. 681) gives micro-photographs of these varieties. Microscopically the masses of Crenothrix are seen enclosed in a gelatinous sheath, in which is imbedded the precipitated metallic hydrate. It is anaërobic and its action is favoured by absence of light. In the absence of dissolved oxygen, the bacillus appears to take its iron from the pipes. Cr. polyspora is found however (“Zeitschrift für analytische Chemie,” XLII. 590) to separate the iron not from the ferrous carbonate (FeCO3) but from iron organically combined. See also Winogradsky, Ueber Eisenbakterien, “Bot. Zeit.,” 1888, and “Chem. Centralbl.” 1904, II. 1332. Transl.]
[17] Wagner in Dingler’s “Polyt. Journal,” CCXVIII. p. 70. Axel Krefting in the above-quoted “Forhandlinger,” p. 4.
[18] Olshausen often noticed on freshly excavated antiquities of various kinds a peculiar smell of resin or gum, especially after treatment with hydrochloric acid (“Verhandl. d. Berl. anthropol. Ges.,” 1884, p. 520). It may be supposed that this odour is due to the traces of hydrocarbons present.
[19] Termed by E. Friedel “Dunstperlen.” “Eintheilungsplan d. Märk. Prov.-Museums,” p. 9.
[20] “Om Konserviring af Jordfundne Jernsager,” in “Aarsberetning fra Foreningen till norske Fortidsmindesmaerkers Bevaring,” 1892, p. 52.
[21] Krause, “Verhandl. d. Berl. anthropol. Ges.,” 1882, p. 533.
[22] Private communication.
[23] “Sitzungsberichte der Alterthumsgesellschaft Prussia,” 1881-2, p. 9.
[24] Merkbuch, Alterthümer aufzugraben und aufzubewahren, 2nd edition, p. 71.
[25] [This fact was noticed by Sir Thomas Browne, 1658, cp. “Hydriotaphia,” cap. iii. Transl.]
[26] In this work the Patina on antiquities only is considered; with that on modern bronzes we are not concerned.
[27] “Mittheilungen d. naturforsch. Gesell. in Bern,” 1865, p. 12.
[28] “Mitth. d. naturforsch. Gesellschaft in Bern,” 1860, p. 69.
[29] “Jahrbuch für Mineralogie,” 1860, p. 813.
[30] Malachite, CuCO3, Cu(OH)2.
[31] Azurite, 2CuCO3, Cu(OH)2.
[32] “Jahrbuch für Mineralogie,” 1865, p. 400.
[33] The extract which here follows is in part verbatim.
[34] “Annalen der Chemie u. Pharmacie,” 1853, Vol. LXXXV. p. 253.
[35] Von Bibra, “Die Bronzen und Kupferlegirungen,” p. 206 et seq.
[36] See the very full quotation from Wibel’s work given above.
[37] I have not been able to find anything on this point in the literature of the subject.
[38] Atacamite, CuCl2, 3Cu(OH)2.
[39] Cf. “Comptes rendus,” 1856, XLIII. p. 735.
[40] “Journal f. praktische Chemie,” XCIV. 1865, p. 314.
[41] “Verhandl. d. Ver. z. Beförd. d. Gewerbf.,” 1869, p. 182.
[42] Dingler, “Polyt. Journal,” 1878, Vol. CCIV. p. 483.
[43] “Berg- u. Hüttenmännische Zeitung,” Vol. XXXVII. 1878, p. 329.
[44] Dingler, “Polyt. Journal,” 1879, Vol. CCXXXII. p. 333.
[45] [Several other analyses of bronzes from various sources have been recently published. Thus Natterer (“Monatshefte für Chemie,” XXI. 256, 1900; also “Chem. Centralblatt,” 1900, I. 1262) examined a corroded bronze statuette from Ephesus. The bronze contained:—Tin 6·09%, lead 4·87%, and copper 89·64%, with traces of zinc.
Bassett (“Proc. Chem. Soc.,” 1903, XIX. 95) gives an analysis of the base of an Egyptian statuette, found in the Nile Delta, probably dating from 200-100 B.C. The base was hollow but filled with lead, and was covered with a thick green coating, which in parts entirely replaced the original metal.
Table I.
| Cu | 50·65% |
| Pb | 6·74% |
| Sn | 2·94% |
| Fe | 0·15% |
| Ni, Mn, etc. | 0·11% |
| Cl | 15·71% |
| SiO2 (as sand) | 1·14% |
| H2O | 11·07% |
| (NH4) | 0·11% |
| 88·62% |
Table II.
| CuCl2 | 29·34% |
| CuO | 46·10% |
| H2O | 11·07% |
| SnO2 | 3·73% |
| PbO | 7·26% |
| Fe2O3 | 0·22% |
| NiO, etc. | 0·14% |
| SiO2 | 1·14% |
| (NH4)Cl | 0·32% |
| 99·32% |
In the second column the chlorine has been calculated as copper chloride, the remaining copper and other metals as oxides.
Traces of calcium were also found, but the amount of sodium was so small that it could only be detected by the flame test. If all the copper had been present as basic chloride (atacamite CuCl2, 3CuO, 3H2O), this would require 26·84% CuCl2, 47·57% CuO, and 10·78% H2O. It would therefore appear that the substance produced by corrosion is less basic than atacamite, and that ammonium chloride may have played a more important part than sodium chloride in the formation of copper chloride, for in this case the sodium was only found in amount too small for estimation.
An analysis of the earliest piece of bronze known, i.e. that from Mêdûm, Egypt (3700 B.C.), gives 8·4% of tin (inner core 9·1%) to 89·8 of copper with a small quantity of arsenic.
An analysis of a celt from the Dowris find (King’s County, Ireland, 1825) gave copper, 85·23; tin 13·11; lead 1·14, with traces of sulphur and carbon. The waste material from the same place yielded 89% copper, 11% tin, with traces only of lead, iron and silver.
On the other hand an early bronze celt (Butterwick, E.R., Yorks.) showed a smaller quantity of tin—10·74%, compared with 87·97% of copper. (Guide to Bronze Age Antiquities, British Museum.)
Mr George Coffey has also published (“Brit. Assoc. Reports,” 1899, p. 873) a tabulated series of analyses of Irish celts which proved to be composed of practically pure copper. Transl.]
[46] Schliemann, “Ilios,” pp. 527 and 571.
[47] “Verhandl. d. Berl. anthropol. Ges.,” 1882, p. 537.
[48] Dingler, “Polyt. Journal,” 1884, Vol. CCLIII. p. 514.
[49] It should be observed that the change in the proportions according to Schuler (see page 24) is only true of the analysis, Column III. In Columns I and II the amount of metallic copper of the patina is indeed smaller, but so is also that of lead and especially of tin. But this may be due to a faulty method in the determination of tin noticed by Olshausen (v. “Verhandl. d. Berl. anthropol. Ges.,” 1897, p. 349). The different proportion of the copper compounds in the patina should also be noted, Schuler giving carbonate and hydrate in the proportion 1:1, Arche and Hassack once as 1:2, and again as the result of two analyses as 1:3.
[50] “Atti della Reale Accademia dei Lincei,” 1893, p. 498.
[51] “Étude sur les métaux découverts dans les fouilles de Dahchour” in “Fouilles à Dahchour.” March-June, 1894, p. 131 et seq. J. de Morgan. See also “Comptes rendus,” 1894, I. 118, p. 768.
[52] “Revue archéologique,” v. 28, 1896, pp. 67 and 202. In the publication for the second half of the same year Lechat maintains the assertion (comp. Elster, p. 23) that in many cases the antique patina is due to the artist.
[53] Ant. Misc. 7382.
[54] J. J. Rein, “Japan,” Vol. II. p. 528.
[55] Ant. Misc. 8579.
[56] According to Graham-Otto, “Lehrbuch der Chemie,” Vol. III. p. 849, cuprous oxide is decomposed by dilute acids which contain oxygen; the cupric oxide is dissolved and metallic copper remains.
[57] Buto, Aeg. 11867.
[58] Ant. Fr. 29.
[59] [The extraordinary deformation produced by this type of patina may be judged from the fact that the features in this instance were so obscured that the nature of the specimen was not recognised and it had accordingly been mounted upside down. Transl.]
[60] Ant. Fr. 53.
[61] Ant. Fr. 53.
[62] Bischoff, “Das Kupfer und seine Legirung,” p. 43. Layard, “Discoveries in the ruins of Nineveh and Babylon,” p. 191. Fellenberg, “Mittheilungen d. naturforsch. Ges. in Bern,” 1860, p. 68.
[63] “Christiania Videnskabs-Selskabs Forhandlinger” for 1892, 16, p. 5.
[64] [Having been broken off and soldered, the base was not subjected to treatment. Transl.]
[65] E. Friedel, “Eintheilungsplan des märk. Provinzialmuseums,” 1882, p. 20.
[66] Aeg. 2348.
[67] Aeg. 13787.
[68] Olshausen, “Verhandl. d. Berl. anthropol. Ges.,” 1884, p. 532, and 1897, pp. 346-7. Kröhnke, “Chem. Untersuchungen an vorgeschichtl. Bronzen Schleswig-Holsteins,” p. 41. See also quotation from Schuler, p. 25.
[69] [This celebrated hoard was found Oct. 9, 1868, on the Galgenberg, near Hildesheim (Hanover), 10 feet below the surface. It consisted of more than 60 pieces, including plates, dishes, tripods, etc., the most notable being a crater, 151⁄ 2 inches in height, ornamented with graceful scroll-work, and a cylix with an Athene in high relief. The workmanship points to a date not later than the first century A.D. Cp. Wieseler, “Der Hildesheimer Silberfund,” Bonn, 1869. Darcel, “Trésor de Hildesheim,” 1870. Transl.]
[71] “Polytechn. Centralblatt,” 1871, p. 916.
[72] “Polytechn. Centralblatt,” 1871, p. 917.
[73] “Berg- u. Hüttenmänn. Zeitung,” 1878, No. 37, p. 327.
[74] Dingler, “Polyt. Journal,” 1871, Vol. CCI. p. 52.
[75] v. E. v. Bibra, “Ueber alte Eisen- u. Silberfunde,” p. 74.
[76] Morgan, “Fouilles à Dahchour,” p. 135.
[77] “Verhandl. d. Berl. anthropol. Ges.,” 1897, p. 348.
[78] Krause, “Verhandl. d. Berl. anthropol. Ges.,” 1883, p. 360.
[79] Muspratt’s “Chemistry,” Vol. III. p. 1389.
[80] “Verhandl. d. Berl. anthropol. Ges.,” 1889, pp. 243 and 244.
[81] Id. 1892, p. 449.
[82] Id. 1897, p. 347.
[83] At that time obtained from the Stralau waterworks.
[84] This was not the well-known Crenothrix only. Cp. “Polytechn. Centralblatt,” 1891-92, p. 195. (See footnote, p. 10.)
[85] It has been found that the formation of this layer of slime may be avoided by the use of tubs which are lined with sheet zinc. The addition of 10-20 cubic centimetres of formalin [40% solution of formaldehyde] to every hundred gallons of water also prevents or restrains the formation of slime. It is not necessary to add formalin each time the water is changed.
[86] Since chlorine compounds (especially common salt) form the predominating substance in the soluble salts contained in limestones their removal may be considered a proof that other salts (e.g. sulphates) have also been removed. Hence it is sufficient to prove the disappearance of chlorine. In the rare cases in which sulphates only are present, a test similar to that mentioned on p. 77, applied to clay objects, should be used. If the water used for soaking salt-containing limestone, earthenware, etc., gives no precipitate, or only turbidity with the silver solution, the determination of chlorine by titration is not applicable.
[87] Though some other kinds of burette may be easier to use, that here recommended (that of Gay-Lussac) is the most convenient for reasons into which we need not enter. The following precautions should be observed: where the burette is not closed by a cork, let a few drops out first to wash away crystals of silver nitrate which may have formed at the mouth. The silver solution should be kept in well stoppered bottles. When filling the burette a glass funnel should be used, so that the cork used for closing the burette is not wetted with the silver solution. Before reading off wait until the level of the fluid is constant, in order that any solution on the sides of the glass tube may have time to run down.
[89] I have found that the amount of chlorine is smaller in winter than in summer. In the summer of 1894, 100 c.c. tap-water from the Stralau Waterworks often required 0·8 c.c. silver solution: but at that time stronger disinfectants were used on account of the cholera, and this may have caused the increase of chlorine; for since then, and even at the present time (winter 1898), 100 c.c. tap-water requires 0·5 to 0·6 c.c. silver solution.
[90] It may be here observed that objects of limestone or of earthenware may be numbered or marked at the back in black iron ink, which does not disappear even after prolonged steeping in water.
[91] Lepsius, “Denkmäler aus Aegypten und Aethiopien.”
[92] It is scarcely necessary to add that any other form of air-pump may be used.
[93] A powerful pressure of water [in combination with a well-acting pump] may cause the fluid to evaporate with such rapidity as to produce bubbles, but these bubbles are easily distinguished by their size from the minute bubbles of air. To avoid this ebullition, the air should not be pumped out too rapidly.
[94] “Merkbuch,” p. 62.
[95] Flinders Petrie, “Archaeological Journal,” Part 45, 1888, p. 88.
[96] Zapon: for further information and references see Appendix.
[97] “Chemische Zeitschrift,” ii. 1903, p. 203.
[98] For particulars of the composition and action of Kessler’s Fluates (salts of Hydrofluosilicic acid) see H. Hauenstein, “Die kessler’schen Fluate” (2nd ed., Berlin, 1895). The depot is “H. Hauenstein, Berlin N. Reinickendorferstrasse, 2b.”
[99] Ger. Patent, No. 31032. The apparatus was one of those used in the moulding room of the Royal Museums for the impregnation of plaster moulds and casts.
[100] In applying the above test it is advisable to add one or two drops of nitric acid before the addition of the barium salt. In this case, too, should any other than distilled water be used for steeping, a preliminary examination should be made to determine the presence or absence of sulphates.
[101] [Pure hydrochloric acid is usually sold in two strengths. Concentrated acid has a strength of about 32%, whilst the “diluted hydrochloric acid” of the Pharmacopœia is about 10%. The former should therefore be diluted with about 15, the latter with about 4 volumes of water. Transl.]
[102] “Chemische Zeitschrift,” II. 1903, p. 761.
[103] [Lecythoi: slender narrow-necked painted vessels which were frequently burnt or buried with the dead; cp. Aristophanes, “Ecclesiazusae,” 996:
ὁς τοῖς νεκροῖσι ζωγραφεῖ τὰς ληκύθους Transl.]
[104] The method of washing objects of unbaked clay suggested by Flinders Petrie in the “Archaeological Journal” (XLV. 1888) is in my experience impracticable.
[105] [Muffle furnaces may be obtained from Messrs Fletcher, Russell and Co., Warrington. If electricity is available, the electric muffle may be used. These may be obtained from Messrs A. Gallenkamp and Co., 19, Sun Street, Finsbury Square, London. The Heat-recorders supplied by H. Watkin, 225, Waterloo Road, Burslem, will be found very convenient in place of Seger’s cones, which may be obtained from Messrs Brady and Martin, Northumberland Road, Newcastle-on-Tyne. Transl.]
[106] “Merkbuch,” p. 78.
[107] [A paraffin prepared from Burmese petroleum. Transl.]
[108] Flinders Petrie, “Archaeological Journal,” XLV. 1888, p. 89.
[109] “Merkbuch,” p. 79.
[110] [It is important to avoid confusion and mistakes arising from the similarity of the terms benzine and benzene.
Benzene (Benzol) is the specific coal-tar product which has the formula C6H6.
Benzine (Benzin) is a light-boiling petroleum distillate, lighter than lamp oil, and with a varying boiling-point. It consists of a number of saturated hydrocarbons of the methane series. It is also called benzoline or petroleum naphtha. Transl.]
[111] Appelgren, Finskt Museum, 1895, p. 56.
[112] A communication from Herr Schjerning, Copenhagen.
[113] Speerschneider, “Antiquarisk Tidsskrift,” 1858-60, p. 178.
[114] Blell, “Sitzungsberichte der Prussia,” 1881-82, p. 24.
[115] Krefting, “Aarsb. fra foreningen t. norske fortidsmindesm. bevar.” 1892, p. 54.
[116] Salzer, “Chem. Zeitung,” XI. 1887, p. 574.
[117] Probably first recommended by Salzer, “Chemiker Zeitung,” XI. 1885, p. 574.
[118] “Kongl. Vitterhets Historie och Antiqvitets Akademiens Månadsblad,” 1885, p. 134.
[120] “Chemiker Zeitung,” XI. 1885, p. 605.
[121] “Zeitschrift f. Ethnologie,” XXXIII. 1902, p. 431, and XXXIV. 1903, p. 791.
[122] “Sitzungsberichte,” 1881-82, p. 10 et seq., and 16 et seq.
[123] In this acid treatment bare hands may be used, but care must be taken to avoid splashing clothes or linen, which will cause red or yellow spots. These are best removed by the immediate application of ammonia, but the yellow spots can only be removed by oxalic acid.
[124] [Germ. “Hammerschlag.” The iron scales which chip off from heated iron at a forge or blacksmith’s shop. Transl.]
[125] No. 17, 1897, p. 333 et seq.
[126] A number of modifications in the metals employed and the composition of the bath have been suggested. Setlik (“Chemiker Zeitung,” XXVII. 1903, p. 454) imbeds iron objects which have a very weak core in a zinc-wire basket immersed in a magma of zinc dust and caustic soda. Personally I should prefer not to attempt a reduction process in such cases, but should rely rather upon mechanical removal of the rust, soaking and impregnation. For the treatment of bronzes this observer prefers the Finkener method (q. v.) and suggests caustic soda, sodium chloride, or ammonia chloride, instead of potassium cyanide as the electrolyte. Rhousopulos (“Chemische Zeitschrift,” II. 1903, pp. 202 and 364) uses zinc and hydrochloric acid, and when dry gives to the bronze a coating of wax. I should deprecate the use of either of these substances, the hydrochloric acid because of the difficulty of completely removing it by steeping and the danger of subsequent decomposition of the bronze, the wax because the contained fatty acids may act upon the metal.
[127] [The period required for complete reduction is, in our experience, often considerably longer. We have sometimes found an 8% soda solution more satisfactory. Transl.]
[128] Krefting’s method affords an excellent illustration of the truth of my remarks in the preface that the literature upon these preservation-methods is very scattered and in consequence has been hitherto but little studied. In 1892, when visiting the Museum at Christiania, I had the opportunity of examining some iron objects which had been treated by Krefting’s method. I then obtained his address, and Herr Krefting kindly communicated his method to me by letter, and in the following year forwarded a reprint of his article referred to above. In 1887 he had described his method to Appelgren by letter, but at that time he treated the object after reduction, washing, and drying, by impregnating it with a paraffin-petroleum solution. In 1897 Appelgren published this method, with drying and impregnation, in ignorance of Krefting’s publication in 1893.
[129] The bottle should not be closed by a glass stopper, but by a rubber bung, or by a cork coated with paraffin or wrapped round with parchment. Soda solution attacks glass, and especially ground glass; thus the stopper may become so firmly fixed into the neck of the bottle as to render its removal impossible.
[130] [Excavated by Dr Thurnam, 1848 (vide “Archaeolog. Journal,” Vol. VI. p. 27). Transl.]
[131] “Chemiker Zeitung,” XI. 1887, p. 605.
[132] Ant. Fr. 1154 a.
[133] I should now use paraffin for impregnation. (Author’s note.)
[134] [Great caution must be used to prevent inhalation of the gas, which is extremely poisonous. Transl.]
[135] Instead of potassium cyanide, I have made experimental use of the much more readily fusible potassium sulphocyanide. This converts the iron compounds into iron sulphide, which is easily got rid of. The sulphide which still adheres to the iron and imparts a not unpleasing blackish colour to the object appears to be stable.
The rest of the treatment is similar to that above described. Having so far only experimented with a few specimens I am not yet in a position to offer any judgment as to the practicability of the process. (Author, 1904.) [For practical objections to this method, which we do not consider satisfactory owing to the instability of the products resulting from the reaction, and the difficulty in removing them by the subsequent washing, see Milbauer, “Zeit. f. anorg. Chem.” XLII. 1904, p. 433 (“Journ. Chem. Soc.” Abstracts, i. 121), where it is stated that treatment of Fe2O3 at 400°C. leads to the formation of K2Fe2S4. Transl.]
[136] Stolba, “Chemiker Zeitung,” XX. 1896, Repertorium, p. 240. [Sodium sulphide has a very deleterious action upon the skin and fingernails which should be protected when using this substance. Transl.]
[137] Flinders Petrie, “Archaeological Journal,” Vol. XLV. 1888, p. 88.
[138] Cp. Mugdan, “Zeit. Elektrochem.” 1903, ix. p. 442.
[139] [A method used by the explorers of the Palestine Exploration Committee for the preservation of much decayed bronzes, as, for instance, those from wells and cisterns, is to place them immediately they are discovered into a strong solution (1 in 10) of carbolic acid. Transl.]
[140] “Merkbuch,” p. 68.
[141] Instead of calcium chloride strong sulphuric acid may be used for dehydration, but the dry chloride is more simple, and less dangerous. If kept in corked bottles, the corks should be covered with paraffin wax to prevent access of moisture.
[143] [The so-called ‘pole paper’ is supplied by most dealers in electrical apparatus. Transl.]
[144] In such a case the hydrated oxide of tin is either present as such in the oxidized bronze, or it is a product of the reduction which has been prevented from falling to the bottom by the high sp. gr. of the potassium cyanide solution. It is also possible that finely divided tin in the reduced bronze may decompose the warm water into oxygen and hydrogen, forming a hydrated oxide of tin. Such a reaction would account for the formation of hydrogen. The hydrogen might at the same time remain occluded until allowed to separate by the cessation of the current and the temperature of the water.
[145] Such, for instance, as is obtained by attaching a suitable nozzle to a fall pipe where there is sufficient water-pressure; v. e.g. “Polytechn. Centralblatt,” 1891-92, p. 199.
[146] I now consider it a better plan not to employ the method of coating with paraffin wax. I thoroughly soak and then dry the reduced bronze with a cloth, and place it in 96% alcohol. This must be renewed after a time, and for large bronzes a third or even a fourth renewal is advisable. The bronze is again wiped and introduced into a drying oven raised to about 212°F. [100°C.]. The unsightly grey colour and rough surface may be much improved by using a brush of the finest wire or very fine emery cloth. The object is finally impregnated with zapon (see Appendix). (Author’s note, 1904.)
[147] [The base was not treated owing to the advanced destruction of the metal. Transl.]
[148] In only about 2% of the bronzes treated in the laboratory of the Royal Museums at Berlin has it been found necessary to interrupt the reduction.
[149] “Polytechn. Centralblatt,” 1891-92, p. 200.
[150] These analyses were made by Schulz in the Laboratory of the Royal Museums.
[151] I quote here the greater part of an article published in Dingler’s “Polytechn. Journal,” 1896, Vol. CCCI. p. 44. The reduction of about 7000 Danish copper coins, undertaken while the above was in the press, gave similarly good results.
[152] The zinc, which in the course of the reduction process may become coated with a thin layer of metallic copper, may be used again. It should be first put through dilute sulphuric acid (in the proportion of 1:2), then washed, rubbed with a steel wire brush and again washed. But it must in such cases be used again while still wet, for if allowed to dry it becomes coated with a layer of oxide and requires to be re-polished.
[153] “Publications de la société pour la recherche et la conservation des monuments historiques dans le grandduché de Luxembourg,” Vol. X. As I have been unable to consult the original, I have here inserted a communication sent to me by Dr Kisa of Cologne. I have tried this method on a few coins.
[154] In another instance—that of a Minotaur group [Ant. Misc. 7382]—the calcium chloride is contained in four shallow glass troughs which are placed round the marble pedestal of the bronze and are loosely covered with a black card.
[155] Grote, “Blätter für Münzkunde,” 1835, I. No. 31, VI.
[156] “Zeitschrift für Numismatik,” Vol. XVII. 1890, p. 100.
[157] “Prometheus,” VIII. 1897, p. 351. A report on the other methods is here given.
[158] “Zeitschrift für Numismatik,” Vol. XX. 1897, p. 325.
[159] “Archaeological Journal,” XLV. 1888, p. 87.
[160] “Riv. Ital. Numism.” 1903, p. 31; also “Chemiker Zeitung,” XXVII. 1903, p. 825.
[161] [In this connection however it must be remembered that celluloid gives off traces of acid for a long time. This may possibly involve risk of injury to certain specimens. Transl.]
[162] “Mittheilungen des Nordböhmischen Gewerbe Museum,” 1903, p. 104.
[163] [A few drops of formalin will serve the same purpose. Transl.]
[164] [Lanoline, especially if applied warm, retains the flexibility of the leather very satisfactorily. It may be here mentioned that the leather of old book-bindings may be preserved by the application, by means of a soft brush, of a mixture of white wax with a small quantity of white vaseline and paraffin wax, brought to a pasty consistency with benzine or turpentine.
The ‘Stearine Glaze’ used for the same purpose is made by boiling one part of caustic soda with eight parts of stearic acid and 50 parts of water till dissolved, then mixing another 150 parts of cold water and stirring until the whole sets to a jelly.
Either of these media should be applied very thinly and then polished with a brush or flannel. If the cover is very bad, considerable improvement is effected by repeated applications of the stearine glaze so as to fill up the damaged surface of the leather. In some cases the addition of some dye such as logwood, or one or other of the acid coal-tar dyes, is advantageous.
Lanoline, or wool fat, i.e. lanoline without the water, is useful, but gives a dull surface to the leather.
In some cases a thin coating of diluted white of egg, to which a few drops of clove oil, or some other essential oil, has been added as an antiseptic is beneficial. Transl.]
[166] “Aarb. for nordisk Oldkynd. og Historie,” 1891, p. 112.
[167] According to an analysis published by L. v. Barth in an account of the collection of papyri belonging to the Archduke Rainer, Part I. p. 120, the salt crystals, after removal of the insoluble constituents, consisted of:
| Potassium sulphate | 0·8% |
| Potassium and sodium chlorides | 92·0% |
| Calcium sulphate | 4·6% |
| Magnesium chloride | 2·8% |
| Organic substances | 0·2% |
[168] “Merkbuch,” p. 60.
[169] Communicated by Herr Leiner of Constance.
[170] “Antiquarisk Tidsskrift,” 1858-60, p. 176.
[171] Id., p. 174.
[172] Olshausen, “Verh. der Berl. anthropol. Ges.” 1885, p. 242, an oral communication from Herr v. Jenner.
[173] Attention may be drawn to a paper which (Dec. 1904) will shortly be published by the Imperial Commission for Monuments of Art and History in Vienna. At a meeting in Vienna a paper was given by Bolle on the animal enemies of paper, leather, and wood, and their destruction by means of carbon bisulphide. Carbon bisulphide is an infallible poison and has no effect upon colours when used in a perfectly dry state. This may be carried out by a preliminary displacement of the air by carbonic acid which is readily obtained in the liquid form in cylinders. Benzine would probably act equally well, but would require a longer time for its action. References to other methods such as the employment of a vacuum or of heat will be found in the same publication.
[174] Keim, “Technische Mittheilungen für Malerei,” 1888, p. 4.
[175] Communication from Herr Straberger of Linz on the Danube.
[176] Communication by Dr Voss.
Dittos and dashes used to represent text have been replaced with the indicated text.
When a scale is given in an image caption, a scale bar has been added to demonstrate the approximate dimensions of the printed image. One centimeter (cm.) and one inch (in.) are depicted in the following format:
Some figure captions have been combined and separated by the word 'and', such that Fig. 44. Fig. 45. becomes Fig. 44. and Fig. 45.
Some presumed printer's errors have been corrected. In particular, the use of c.c. has been normalized when periods were missing, the degree symbol (°) has been added when it appears to have been missing, and words and numbers in the Index and Table of Contents were changed to match the spelling and placement in the body of the text. Some additional presumed errors which have been corrected are listed below with the original text (top) and the replacement text (bottom):
Literature xiv Table of Contents
Literature xiii
vesse p. 59
vessel
Gay Lussac p. 62
Gay-Lussac
16 Feb 1904 p. 65
16 Feb 1894
Konigsberg p. 102
Königsberg
Royal Musums p. 154
Royal Museums
"Lexikon d. gesamsen Technik," Footnote 1
"Lexikon d. gesamten Technik,"
End of the Project Gutenberg EBook of The Preservation of Antiquities, by
Friedrich Rathgen
*** END OF THIS PROJECT GUTENBERG EBOOK THE PRESERVATION OF ANTIQUITIES ***
***** This file should be named 46851-h.htm or 46851-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/6/8/5/46851/
Produced by Chris Curnow, Sonya Schermann and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)
Updated editions will replace the previous one--the old editions
will be renamed.
Creating the works from public domain print editions means that no
one owns a United States copyright in these works, so the Foundation
(and you!) can copy and distribute it in the United States without
permission and without paying copyright royalties. Special rules,
set forth in the General Terms of Use part of this license, apply to
copying and distributing Project Gutenberg-tm electronic works to
protect the PROJECT GUTENBERG-tm concept and trademark. Project
Gutenberg is a registered trademark, and may not be used if you
charge for the eBooks, unless you receive specific permission. If you
do not charge anything for copies of this eBook, complying with the
rules is very easy. You may use this eBook for nearly any purpose
such as creation of derivative works, reports, performances and
research. They may be modified and printed and given away--you may do
practically ANYTHING with public domain eBooks. Redistribution is
subject to the trademark license, especially commercial
redistribution.
*** START: FULL LICENSE ***
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full Project
Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project Gutenberg-tm
electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or destroy
all copies of Project Gutenberg-tm electronic works in your possession.
If you paid a fee for obtaining a copy of or access to a Project
Gutenberg-tm electronic work and you do not agree to be bound by the
terms of this agreement, you may obtain a refund from the person or
entity to whom you paid the fee as set forth in paragraph 1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this agreement
and help preserve free future access to Project Gutenberg-tm electronic
works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the Foundation"
or PGLAF), owns a compilation copyright in the collection of Project
Gutenberg-tm electronic works. Nearly all the individual works in the
collection are in the public domain in the United States. If an
individual work is in the public domain in the United States and you are
located in the United States, we do not claim a right to prevent you from
copying, distributing, performing, displaying or creating derivative
works based on the work as long as all references to Project Gutenberg
are removed. Of course, we hope that you will support the Project
Gutenberg-tm mission of promoting free access to electronic works by
freely sharing Project Gutenberg-tm works in compliance with the terms of
this agreement for keeping the Project Gutenberg-tm name associated with
the work. You can easily comply with the terms of this agreement by
keeping this work in the same format with its attached full Project
Gutenberg-tm License when you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are in
a constant state of change. If you are outside the United States, check
the laws of your country in addition to the terms of this agreement
before downloading, copying, displaying, performing, distributing or
creating derivative works based on this work or any other Project
Gutenberg-tm work. The Foundation makes no representations concerning
the copyright status of any work in any country outside the United
States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other immediate
access to, the full Project Gutenberg-tm License must appear prominently
whenever any copy of a Project Gutenberg-tm work (any work on which the
phrase "Project Gutenberg" appears, or with which the phrase "Project
Gutenberg" is associated) is accessed, displayed, performed, viewed,
copied or distributed:
This eBook is for the use of anyone anywhere at no cost and with
almost no restrictions whatsoever. You may copy it, give it away or
re-use it under the terms of the Project Gutenberg License included
with this eBook or online at www.gutenberg.org
1.E.2. If an individual Project Gutenberg-tm electronic work is derived
from the public domain (does not contain a notice indicating that it is
posted with permission of the copyright holder), the work can be copied
and distributed to anyone in the United States without paying any fees
or charges. If you are redistributing or providing access to a work
with the phrase "Project Gutenberg" associated with or appearing on the
work, you must comply either with the requirements of paragraphs 1.E.1
through 1.E.7 or obtain permission for the use of the work and the
Project Gutenberg-tm trademark as set forth in paragraphs 1.E.8 or
1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any additional
terms imposed by the copyright holder. Additional terms will be linked
to the Project Gutenberg-tm License for all works posted with the
permission of the copyright holder found at the beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including any
word processing or hypertext form. However, if you provide access to or
distribute copies of a Project Gutenberg-tm work in a format other than
"Plain Vanilla ASCII" or other format used in the official version
posted on the official Project Gutenberg-tm web site (www.gutenberg.org),
you must, at no additional cost, fee or expense to the user, provide a
copy, a means of exporting a copy, or a means of obtaining a copy upon
request, of the work in its original "Plain Vanilla ASCII" or other
form. Any alternate format must include the full Project Gutenberg-tm
License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works provided
that
- You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is
owed to the owner of the Project Gutenberg-tm trademark, but he
has agreed to donate royalties under this paragraph to the
Project Gutenberg Literary Archive Foundation. Royalty payments
must be paid within 60 days following each date on which you
prepare (or are legally required to prepare) your periodic tax
returns. Royalty payments should be clearly marked as such and
sent to the Project Gutenberg Literary Archive Foundation at the
address specified in Section 4, "Information about donations to
the Project Gutenberg Literary Archive Foundation."
- You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or
destroy all copies of the works possessed in a physical medium
and discontinue all use of and all access to other copies of
Project Gutenberg-tm works.
- You provide, in accordance with paragraph 1.F.3, a full refund of any
money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days
of receipt of the work.
- You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project Gutenberg-tm
electronic work or group of works on different terms than are set
forth in this agreement, you must obtain permission in writing from
both the Project Gutenberg Literary Archive Foundation and Michael
Hart, the owner of the Project Gutenberg-tm trademark. Contact the
Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
public domain works in creating the Project Gutenberg-tm
collection. Despite these efforts, Project Gutenberg-tm electronic
works, and the medium on which they may be stored, may contain
"Defects," such as, but not limited to, incomplete, inaccurate or
corrupt data, transcription errors, a copyright or other intellectual
property infringement, a defective or damaged disk or other medium, a
computer virus, or computer codes that damage or cannot be read by
your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium with
your written explanation. The person or entity that provided you with
the defective work may elect to provide a replacement copy in lieu of a
refund. If you received the work electronically, the person or entity
providing it to you may choose to give you a second opportunity to
receive the work electronically in lieu of a refund. If the second copy
is also defective, you may demand a refund in writing without further
opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO OTHER
WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of damages.
If any disclaimer or limitation set forth in this agreement violates the
law of the state applicable to this agreement, the agreement shall be
interpreted to make the maximum disclaimer or limitation permitted by
the applicable state law. The invalidity or unenforceability of any
provision of this agreement shall not void the remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in accordance
with this agreement, and any volunteers associated with the production,
promotion and distribution of Project Gutenberg-tm electronic works,
harmless from all liability, costs and expenses, including legal fees,
that arise directly or indirectly from any of the following which you do
or cause to occur: (a) distribution of this or any Project Gutenberg-tm
work, (b) alteration, modification, or additions or deletions to any
Project Gutenberg-tm work, and (c) any Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of computers
including obsolete, old, middle-aged and new computers. It exists
because of the efforts of hundreds of volunteers and donations from
people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future generations.
To learn more about the Project Gutenberg Literary Archive Foundation
and how your efforts and donations can help, see Sections 3 and 4
and the Foundation information page at www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive
Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg
Literary Archive Foundation are tax deductible to the full extent
permitted by U.S. federal laws and your state's laws.
The Foundation's principal office is located at 4557 Melan Dr. S.
Fairbanks, AK, 99712., but its volunteers and employees are scattered
throughout numerous locations. Its business office is located at 809
North 1500 West, Salt Lake City, UT 84116, (801) 596-1887. Email
contact links and up to date contact information can be found at the
Foundation's web site and official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To
SEND DONATIONS or determine the status of compliance for any
particular state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations.
To donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic
works.
Professor Michael S. Hart was the originator of the Project Gutenberg-tm
concept of a library of electronic works that could be freely shared
with anyone. For forty years, he produced and distributed Project
Gutenberg-tm eBooks with only a loose network of volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as Public Domain in the U.S.
unless a copyright notice is included. Thus, we do not necessarily
keep eBooks in compliance with any particular paper edition.
Most people start at our Web site which has the main PG search facility:
www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.